A Novel Nonenzymatic Hydrogen Peroxide Sensor Based on a Polypyrrole Nanowire-Copper Nanocomposite Modified Gold Electrode
Abstract
:1. Introduction
2. Results and Discussion
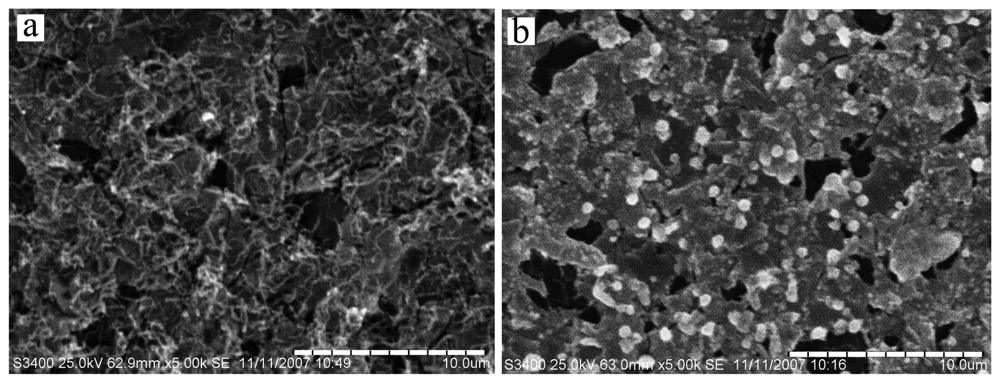
2.1 Characterization of Electrode Surface
2.2 Electrochemical Characterization of the Modified Electrode
2.3 Influence of Potential on Sensor Response
2.4 Optimization of the Concentration of NaOH for the Sensor
2.5 The Sensor Response to Hydrogen Peroxide
2.6 Stability of the Hydrogen Peroxide Sensor
2.7 Selectivity of the Hydrogen Peroxide Sensor
2.8 Recovery Experiment
3. Experimental Section
3.1 Reagents
3.2 Apparatus and Chemicals
3.3 The modification of the Electrode
4. Conclusions
Acknowledgments
References and Notes
- Bartlett, P.N.; Birkin, P.R.; Wang, J.H.; Palmisano, F.; Benedetto, G.D. An enzyme switch employing direct electrochemical communication between horseradish peroxidase and a poly (aniline) film. Anal. Chem. 1998, 70, 3685–3694. [Google Scholar]
- Wang, J.; Lin, Y.H.; Chen, L. Organic-phase biosensor for monitoring phenol and hydrogen peroxide in pharmaceutical antibacterial products. Analyst 1993, 118, 277–280. [Google Scholar]
- Sellers, R.M. Spectropohotometric determination of hydrogen peroxide using potassium titanium (IV) oxalate. Analyst 1980, 105, 950–954. [Google Scholar]
- Nakabayashi, Y.; Yoshikawa, H. Amperometric Biosensors for Sensing of Hydrogen Peroxide Based on Electron Transfer between Horseradish Peroxidase and Ferrocene as a Mediator. Anal. Sci. 2000, 16, 609–614. [Google Scholar]
- Matsubara, C.; Kawamoto, N.; Takamura, K. Oxo[5,10,15,20-tetra (4-pyridyl)porphyrinato] titanium (IV): an ultra-high sensitivity spectrophotometric reagent for hydrogen peroxide. Analyst 1992, 117, 1781–1784. [Google Scholar]
- Hanaoka, S.; Lin, J.M.; Yamada, M. Chemiluminescent flow sensor for H2O2 based on the decomposition of H2O2 catalyzed by cobalt (II)-ethanolamine complex immobilized on resin. Anal. Chim. Acta 2001, 426, 57–64. [Google Scholar]
- Li, J.; Tan, S.N.; Ge, H.L. Silica sol–gel immobilized amperometric biosensor for hydrogen peroxide. Anal. Chim. Acta 1996, 335, 137–145. [Google Scholar]
- Garguilo, M.G.; Huynh, N.; Proctor, A.; Michael, A.C. Amperometric sensors for peroxide, choline, and acetylcholine based on electron transfer between horseradish peroxidase and a redox polymer. Anal. Chem. 1993, 65, 523–528. [Google Scholar]
- Xiao, Y.; Ju, H.X.; Chen, H.Y. A reagentless hydrogen peroxide sensor based on incorporation of horseradish peroxidase in poly (thionine) film on a monolayer modified electrode. Anal. Chim. Acta 1999, 391, 299–306. [Google Scholar]
- Xiao, Y.; Ju, H.X.; Chen, H.Y. Direct electrochemistry of horseradish peroxidase immobilized on a colloid/cysteamine-modified gold electrode. Anal. Biochem. 2000, 278, 22–28. [Google Scholar]
- Zhang, J.D.; Oyama, M. A hydrogen peroxide sensor based on the peroxidase activity of hemoglobin immobilized on gold nanoparticles-modified ITO electrode. Electrochim. Acta 2004, 50, 85–90. [Google Scholar]
- Wang, Q.L.; Lu, G.X.; Yang, B.J. Hydrogen peroxide biosensor based on direct electrochemistry of hemoglobin immobilized on carbon paste electrode by a silica sol–gel film. Sens. Actuat. B 2004, 99, 50–57. [Google Scholar]
- Liu, X.J.; Chen, T.; Liu, L.F.; Li, G.X. Electrochemical characteristics of heme proteins in hydroxyethylcellulose film. Sens. Actuators B 2006, 11, 106–111. [Google Scholar]
- Feng, J.J.; Zhao, G.; Xu, J.J.; Chen, H.Y. Direct electrochemistry and electrocatalysis of heme proteins immobilized on gold nanoparticles stabilized by chitosan. Anal. Biochem. 2005, 34, 2280–2286. [Google Scholar]
- Yang, Y.; Mu, S. Bioelectrochemical responses of the polyaniline horseradish peroxidase electrodes. J. Electroanal. Chem 1997, 432, 71–78. [Google Scholar]
- Wittstock, G.; Strubing, A.; Szargan, R.; Werner, G. Glucose oxidation at bismuth-modified platinum electrodes. J. Electroanal. Chem. 1998, 444, 61–73. [Google Scholar]
- Fumiyo, K.; Satoshi, K. Electrocatalytic activity of bamboo-Structured carbon nanotubes paste electrode toward hydrogen peroxide. Anal. Lett 2006, 39, 903–911. [Google Scholar]
- Lu, Q.; Zhou, T.; Hu, S.S. Direct electrochemistry of hemoglobin in PHEA and its catalysis to H2O2. Biosens. Bioelectron 2007, 22, 899–904. [Google Scholar]
- Ashwell, G.J. Molecular Electronics; John Wiley and Sons Ltd: New York, 1992. [Google Scholar]
- Shin, M.C.; Kim, H.S. Effects of enzyme concentration and film thickness on the analytical performance of a polypyrrole/glucose oxidase biosensor. Anal. Lett 1995, 28, 1017–1031. [Google Scholar]
- Fiorito, P. A.; Brett, C.M.A.; Torresi, S.C. Polypyrrole/copper hexacyanoferrate hybrid as redox mediator for glucose biosensors. Talanta 2006, 69, 403–408. [Google Scholar]
- Strike, D.J.; Rooij, N. F. D.; Koudelka-Hep, M.; Ulmann, M.; Augustynski, J. Electrocatalytic oxidation of methanol on platinum microparticles in polypyrrole. J. Appl. Electrochem. 1992, 22, 922–926. [Google Scholar]
- Rau, J.R.; Chen, S.C.; Sun, H.W. Characterization of a polypyrrole microsensor for nitrate and nitrite ions. Electrochim. Acta 1994, 39, 2773–2779. [Google Scholar]
- Li, J.; Lin, X.Q. Glocose biosensor based on immobilization of glucose oxidase in poly(o-aminophenol) film on polypyrrole-Pt nanocomposite modified glassy carbon electrode. Biosens. Bioelectron 2007, 22, 2898–2905. [Google Scholar]
- Chen, W.; Li, C.M. Electrosynthesis and characterization of polypyrrole/Au nanocomposite. Electrochim. Acta 2007, 52, 2845–2845. [Google Scholar]
- Bose, C.S.C.; Rajeshwar, K. Efficient electrocatalyst assemblies for proton and oxygen reduction: the electrosynthesis and characterization of polypyrrole films containing nanodispersed platinum particle. J. Electroanal. Chem. 1992, 333, 235–256. [Google Scholar]
- Liu, Y.C.; Lee, H.T.; Yang, S.J. Strategy for the syntheses of isolated fine silver nanoparticles and polypyrrole/silver nanocomposites on gold substrates. Electrochim. Acta 2006, 51, 3441–3445. [Google Scholar]
- Roux, S.; Soler-Illia, G.J.; Champagne, S.; Audebert, P.; Sanchez, C. Titania /Polypyrrole Hybrid Nanocomposites Built from In-Situ Generated Organically Functionalized Nanoanatase Building Blocks. Adv. Mater. 2003, 15, 217–221. [Google Scholar]
- Cioffi, N.; Torsi, L.; Losito, I.; Franco, C. D.; Bari, I.D.; Chiavarone, L.; Scamarcio, G.; Tsakova, V.; Sabbatini, L.; Zambonin, P.G. Electrosynthesis and analytical characterization of polypyrrole thin films modified with copper nanoparticles. J. Mater. Chem. 2001, 11, 1434–1440. [Google Scholar]
- Li, J.; Lin, X.Q. Electrocatalytic reduction of nitrite at polypyrrole nanowire– platinum nanocluster modified glassy carbon electrode. Microchem. J. 2007, 87, 41–46. [Google Scholar]
- Tian, Y.; Wang, J.X.; Wang, Z.; Wang, S.C. Solid-phase extraction and amperometric determination of nitrite with polypyrrole nanowire modified electrodes. Sens. Actuat. B 2005, 104, 23–28. [Google Scholar]
- Gu, T.T.; Hasebe, Y. DNA–Cu (II) poly(amine) complex membrane as novel catalytic layer for highly sensitive amperometric determination of hydrogen peroxide. Biosens. Bioelectron. 2006, 21, 2121–2128. [Google Scholar]
- Farrell, S. T.; Breslin, C. B. Oxidation and photo-induced oxidation of glucose at a polyaniline film modified by copper particles. Electrochim. Acta 2004, 49, 4497–4503. [Google Scholar]
- Kang, X.H.; Mai, Z.B.; Zou, X.Y.; Cai, P.X.; Mo, J.Y. A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal. Bioanalytical. Chem. 2007, 363, 143–150. [Google Scholar]
- Xu, Q.; Zhao, Y.; Xu, J.Z. Preparation of functionalized copper nanoparticles and fabrication of a glucose sensor. Sens. Actuat. B 2006, 114, 379–386. [Google Scholar]
- Cioffi, N.; Torsi, L.; Sabbatini, L.; Zambonin, P.G.; Bleve-Zacheo, T. Electrosynthesis and characterisation of nanostructured palladium–polypyrrole composites. J. Electroanal.Chem. 2000, 488, 42–47. [Google Scholar]
- Chen, S.H.; Yuan, R.; Chai, Y.Q.; Zhang, L.Y.; Wang, N.; Li, X.L. Amperometric third-generation hydrogen peroxide biosensor based on the immobilization of hemoglobin on multiwall carbon nanotubes and gold colloidal nanoparticles. Biosens. Bioelectron. 2007, 22, 1268–1274. [Google Scholar]
- Wang, F.C.; Yuan, R.; Chai, Y.Q.; Tang, D.P. Probing traces of hydrogen peroxide by use of a biosensor based on mediator-free DNA and horseradish peroxidase immobilized on silver nanoparticles. Anal. Bioanal. Chem. 2007, 387, 709–717. [Google Scholar]





| Interfering reagent | Current ratioa,b |
|---|---|
| glucose | 1.02 |
| glycine | 1.01 |
| ethanol | 1.01 |
| acetic acid | 0.99 |
| l-cysteine | 0.96 |
| Sample H2O2 (mmol L-1) | Added H2O2 (mmol L-1) | Detected H2O2 (mmol L-1) | Recovery (%) |
|---|---|---|---|
| 0.08 | 0.024 | 0.106 | 101.9 |
| 0.4 | 0.35 | 0.726 | 96.8 |
| 1.2 | 0.4 | 1.68 | 105 |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, T.; Yuan, R.; Chai, Y.; Li, W.; Ling, S. A Novel Nonenzymatic Hydrogen Peroxide Sensor Based on a Polypyrrole Nanowire-Copper Nanocomposite Modified Gold Electrode. Sensors 2008, 8, 5141-5152. https://doi.org/10.3390/s8085141
Zhang T, Yuan R, Chai Y, Li W, Ling S. A Novel Nonenzymatic Hydrogen Peroxide Sensor Based on a Polypyrrole Nanowire-Copper Nanocomposite Modified Gold Electrode. Sensors. 2008; 8(8):5141-5152. https://doi.org/10.3390/s8085141
Chicago/Turabian StyleZhang, Tingting, Ruo Yuan, Yaqin. Chai, Wenjuan Li, and Shujuan Ling. 2008. "A Novel Nonenzymatic Hydrogen Peroxide Sensor Based on a Polypyrrole Nanowire-Copper Nanocomposite Modified Gold Electrode" Sensors 8, no. 8: 5141-5152. https://doi.org/10.3390/s8085141




