Fabrication of Pillar Shaped Electrode Arrays for Artificial Retinal Implants
Abstract
:1 Introduction
2. Methods
2.1. Fabrication of the microelectrode array
2.2. Evaluation of the microelectrode array
2.3. Electrophysiological tests
3. Results and Discussion
3.1 Fabrication result
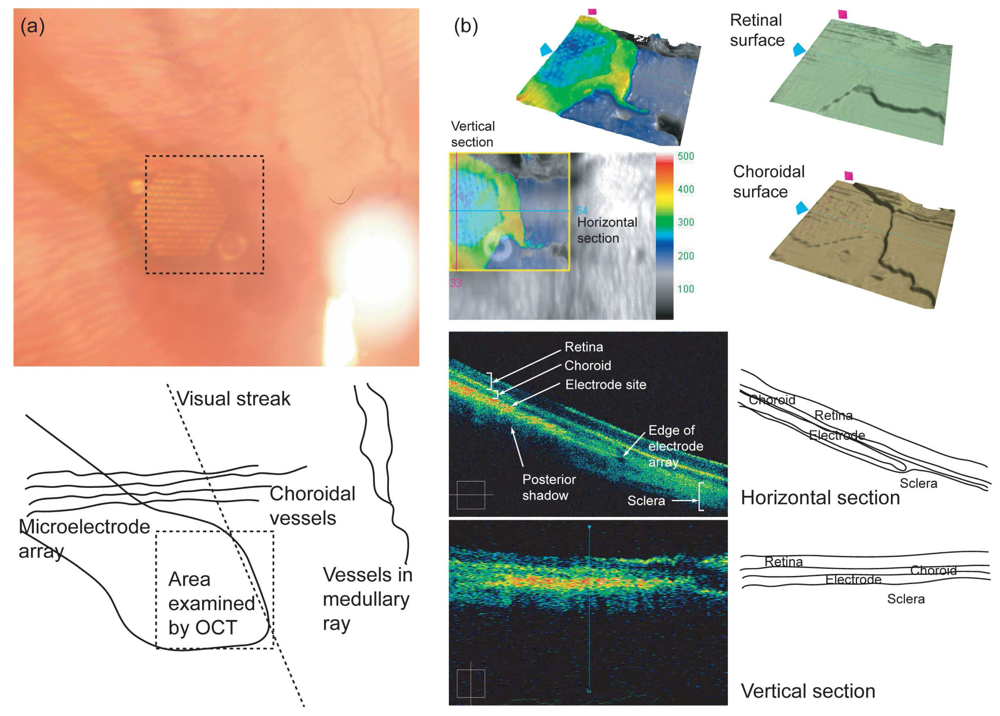
3.2 Evaluation of the microelectrode array
3.3 Electrophysiological test
3.4 General discussion
4. Conclusions
Acknowledgments
References
- Brelen, M.E.; Duret, F.; Gerard, B.; Delbeke, J.; Veraart, C. Creating a meaningful visual perception in blind volunteers by optic nerve stimulation. J. Neural Eng. 2005, 2, S22–S28. [Google Scholar]
- Humayun, M.S.; Weiland, J.D.; Fujii, G.U.; Greenberg, R.; Williamson, R.; Little, J.; Mech, B.; Cimmarusti, V.; Boemel, G.; Dagnelie, G.; Juan, E. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 2003, 43, 2573–2581. [Google Scholar]
- Richard, G.; Keserue, M.; Feucht, M.; Post, N.; Hornig, R. Visual Perception after long-term implantation of a retinal implant. Invest. Ophthalmol. Vis. Sci. 2008, 49, E-Abstract. 1786. [Google Scholar]
- Rizzo, J.F.; Wyatt, J.; Loewenstein, J.; Kelly, S.; Shire, D. Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. Invest. Ophthalmol. Vis. Sci. 2003, 44, 5355–5361. [Google Scholar]
- Zrenner, E. Will retinal implants restore vision? Science 2002, 295, 1022–1025. [Google Scholar]
- Peachey, N.S.; Chow, A.Y. Subretinal implantation of semiconductor-based photodiodes: progress and challenges. J. Rehab. Res. Dev. 1999, 36, 371–376. [Google Scholar]
- Normann, R.A.; Maynard, E.M.; Guillory, K.S.; Warren, D.J. Cortical implants for the blind. IEEE Spectr. 1996, 33, 54–59. [Google Scholar]
- Eckhorn, R.; Wilms, M.; Schanze, T.; Eger, M.; Hesse, L.; Eysel, U.T.; Kisvarday, Z.F.; Zrenner, E.; Gekeler, F.; Schwahn, H.; Shinoda, K.; Sachs, H.; Walter, P. Visual resolution with retinal implants estimated from recordings in cat visual cortex. Vision Res. 2006, 46, 2675–2690. [Google Scholar]
- Cha, K.; Horch, K.W.; Normann, R.A. Mobility Performance with a Pixelized Vision System. Vision Res. 1992, 32, 1367–1372. [Google Scholar]
- Geruschat, D.R.; Turano, K.A.; Stahl, J.W. Traditional Measures of Mobility Performance and Retinitis Pigmentosa. Optom. Vis. Sci. 1998, 75, 525–537. [Google Scholar]
- Sommerhalder, J.; Oueghlani, E.; Bagnoud, M.; Leonards, U.; Safran, A.B.; Pelizzone, M. Simulation of artificial vision: I. Eccentric reading of isolated words, and perceptual learning. Vision Res. 2003, 43, 269–283. [Google Scholar]
- Sommerhalder, J.; Rappaz, B.; Haller, R.; Fornos, A.P.; Safran, A.B.; Pelizzone, M. Simulation of artificial vision: II. Eccentric reading of full-page text and the learning of this task. Vision Res. 2004, 44, 1693–1706. [Google Scholar]
- Scribner, D.; Humayun, M.; Justus, B.; Merritt, C.; Klein, R.; Howard, J.G.; Peckerar, M.; Perkins, F.; Johnson, L.; Bassett, W.; Skeath, P.; Margalit, E.; Kah-Guan, Au E.; Weiland, J.; de Juan, E.; Finch, J.; Graham, R.; Trautfield, C.; Taylor, S. Intraocular retinal prosthesis test device. The 23rd Annual EMBS International Conference, Istanbul, Turkey, October 25-28; 25-28 October 2001; pp. 3431–3435. [Google Scholar]
- Hung, A.; Zhou, D.; Greenberg, R.; Judy, J.W. Micromachined Electrodes for Retinal Prostheses. The 2nd Annual International IEEE -EMBS Special Topic Conference on Microtechnologies in Medicine & Biology, Wisconsin, USA, May 2-4, 2002; pp. 76–79.
- Hungar, K.; Goertz, M.; Slavcheva, E.; Spanier, G.; Weidig, C.; Mokwa, W. Production Processes for a Flexible Retina Implant. Sens. Actuat. A. 2005, 123-124, 172–178. [Google Scholar]
- Bhandari, R.; Negi, S.; Rieth, L.; Normann, R.A.; Solzbacher, F. A Novel Method of Fabricating Convoluted Shaped Electrode Arrays for Neural and Retinal Prosthesis. In Transducers '07.; Lyon, France, June 10-14 2007; pp. 1251–1254. [Google Scholar]
- Palanker, D.; Vankov, A.; Huie, P.; Baccus, S. Design of a high-resolution optoelectronic retinal prosthesis. J. Neural. Eng. 2005, 2, S105–S120. [Google Scholar]
- Kim, E.T.; Seo, J.M.; Zhou, J.A.; Jung, H.; Kim, S.J. A retinal implant technology based on flexible polymer electrode and optical/electrical stimulation. IEEE International Workshop on Biomedical Circuits & Systems, Singapore, Dec. 1-3, 2004.
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar]
- Suaning, G.J.; Hallum, L.E.; Preston, P.J.; Lovell, N.H. An Efficient multiplexing method for addressing large numbers of electrodes in a visual neuroprosthesis. Proceedings of the 26th Annual International Conference of the IEEE EMBS, San Francisco, Sep. 1-5, 2004.
- Okuno, T.; Oku, H.; Ikeda, T. The reproducibility and sensitivity of visual evoked potential testing in rabbits. Neuro-Ophthalmol. 2002, 26, 59–66. [Google Scholar]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar]




© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, E.T.; Seo, J.-M.; Woo, S.J.; Zhou, J.A.; Chung, H.; Kim, S.J. Fabrication of Pillar Shaped Electrode Arrays for Artificial Retinal Implants. Sensors 2008, 8, 5845-5856. https://doi.org/10.3390/s8095845
Kim ET, Seo J-M, Woo SJ, Zhou JA, Chung H, Kim SJ. Fabrication of Pillar Shaped Electrode Arrays for Artificial Retinal Implants. Sensors. 2008; 8(9):5845-5856. https://doi.org/10.3390/s8095845
Chicago/Turabian StyleKim, Eui Tae, Jong-Mo Seo, Se Joon Woo, Jing Ai Zhou, Hum Chung, and Sung June Kim. 2008. "Fabrication of Pillar Shaped Electrode Arrays for Artificial Retinal Implants" Sensors 8, no. 9: 5845-5856. https://doi.org/10.3390/s8095845




