Human NK Cell Up-regulation of CD69, HLA-DR, Interferon γ Secretion and Cytotoxic Activity by Plasmacytoid Dendritic Cells is Regulated through Overlapping but Different Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1 Cell isolation (pDC and NK cells)
2.2 Co-cultures
2.3 Phenotypic characterisation
2.4 Intracellular detection of IFNγ
2.5 Detection of CD107a on NK cells
2.6 ELISA assays
2.7 Cell mediated cytotoxicity assay
3. Results
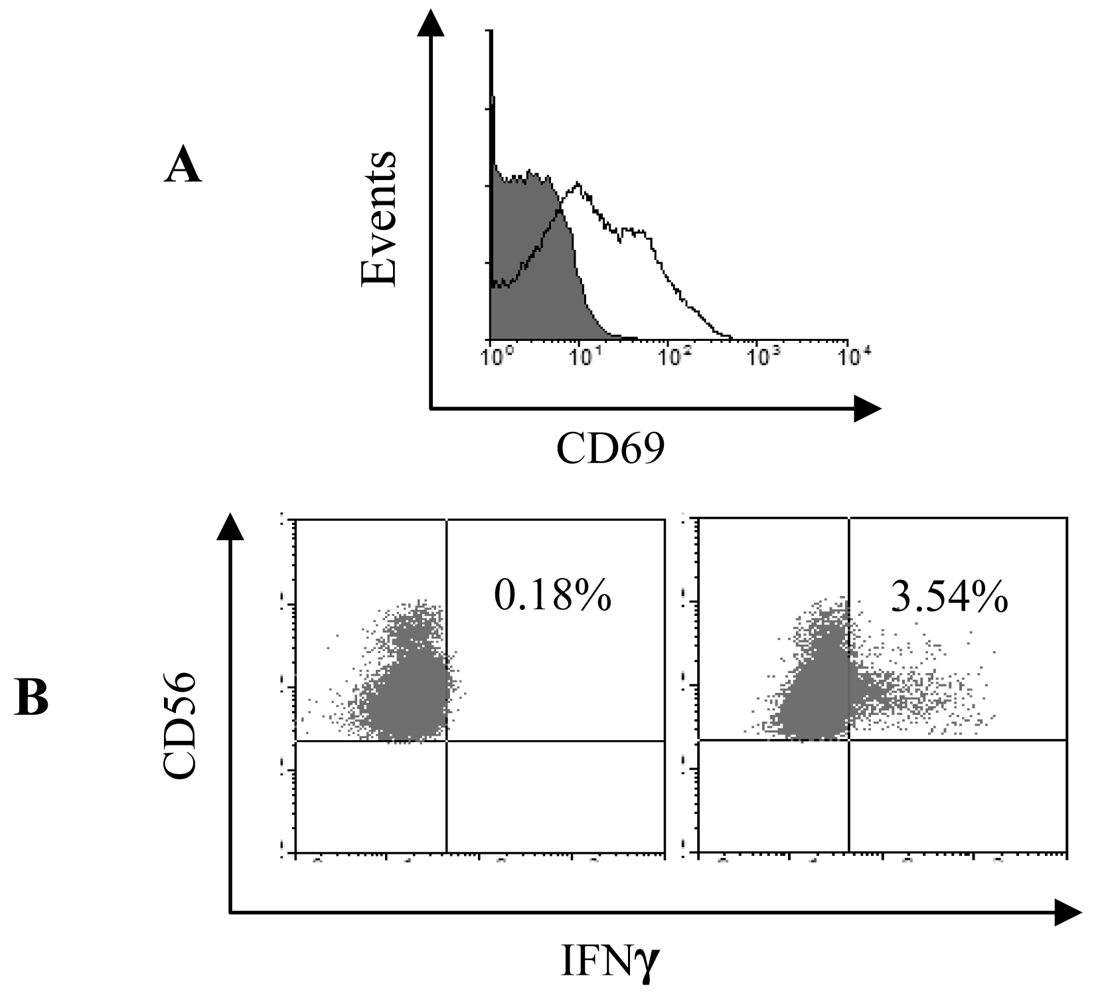
3.1 Purity of the pDC and NK populations
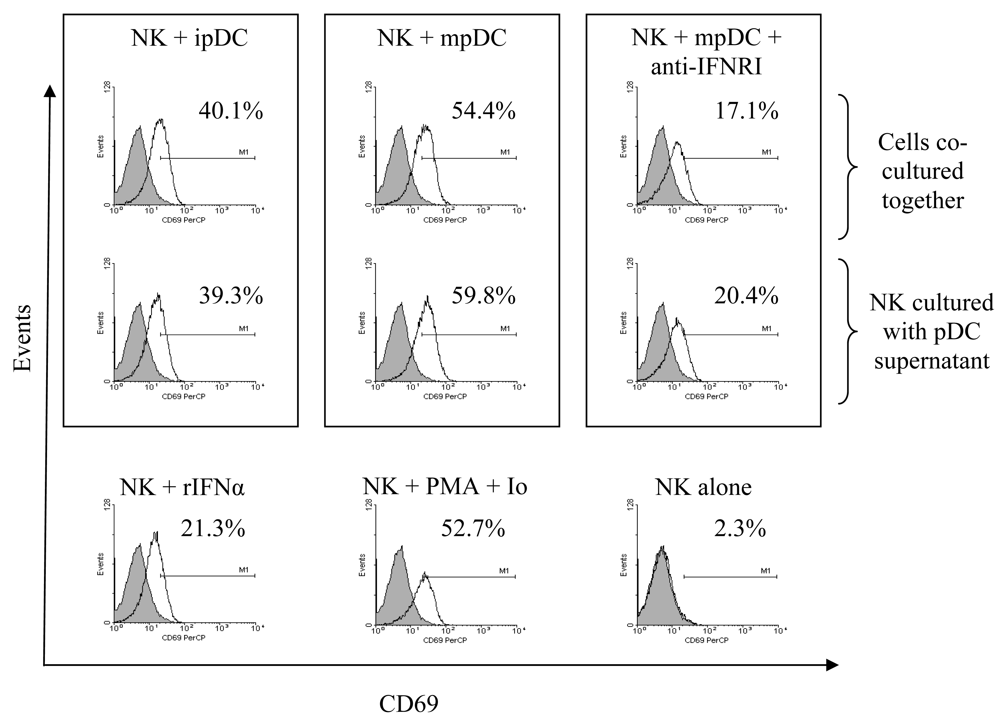
3.2 pDC induce CD69 up-regulation on NK cells in a cell-contact independent, type I interferon dependent mechanism
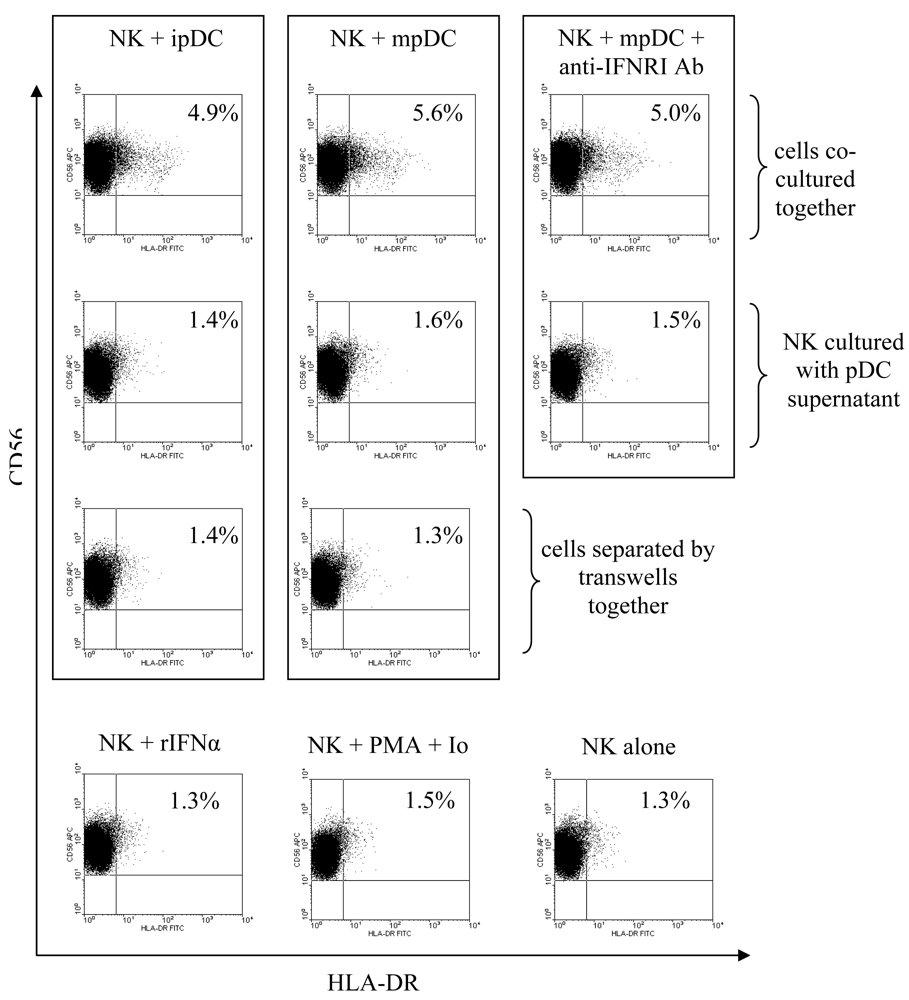
3.3 pDC induce upregulation of HLA-DR on NK cells through cellular contact
3.4 Mature pDC induce IFNγ secretion by NK cells via signalling through the type I interferon receptor
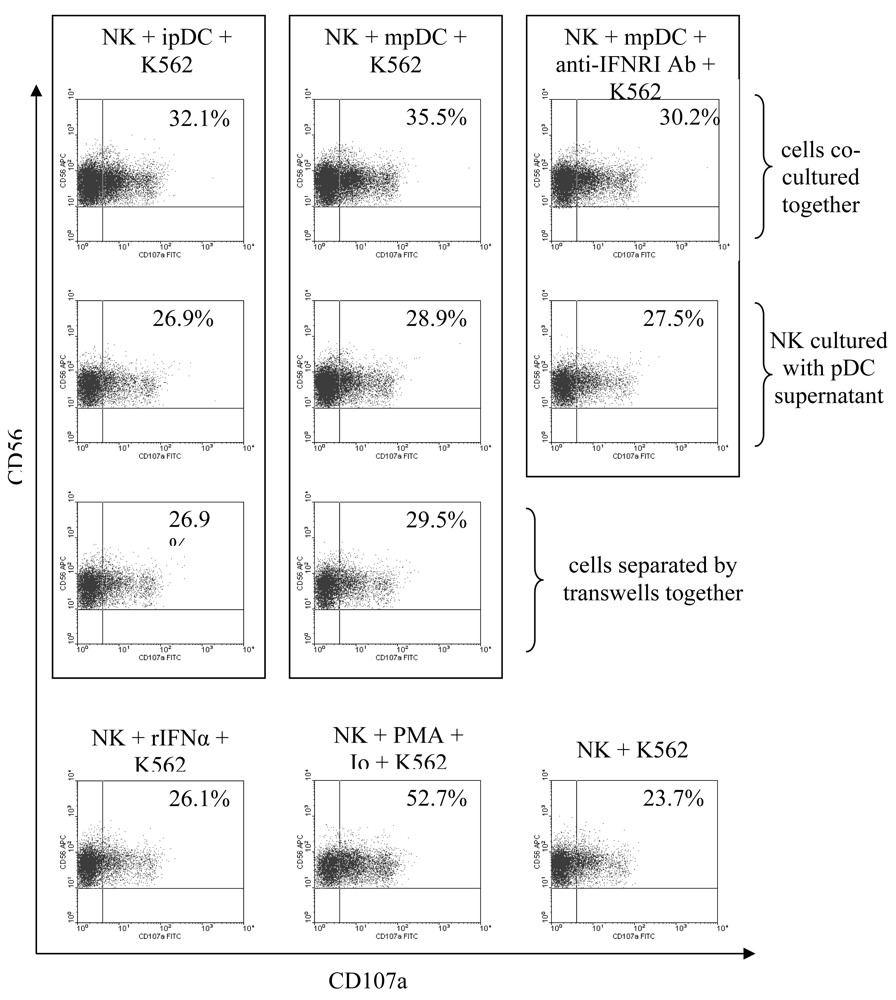
3.5 NK cell degranulation is induced by type I interferons and enhanced by cellular contact with pDC
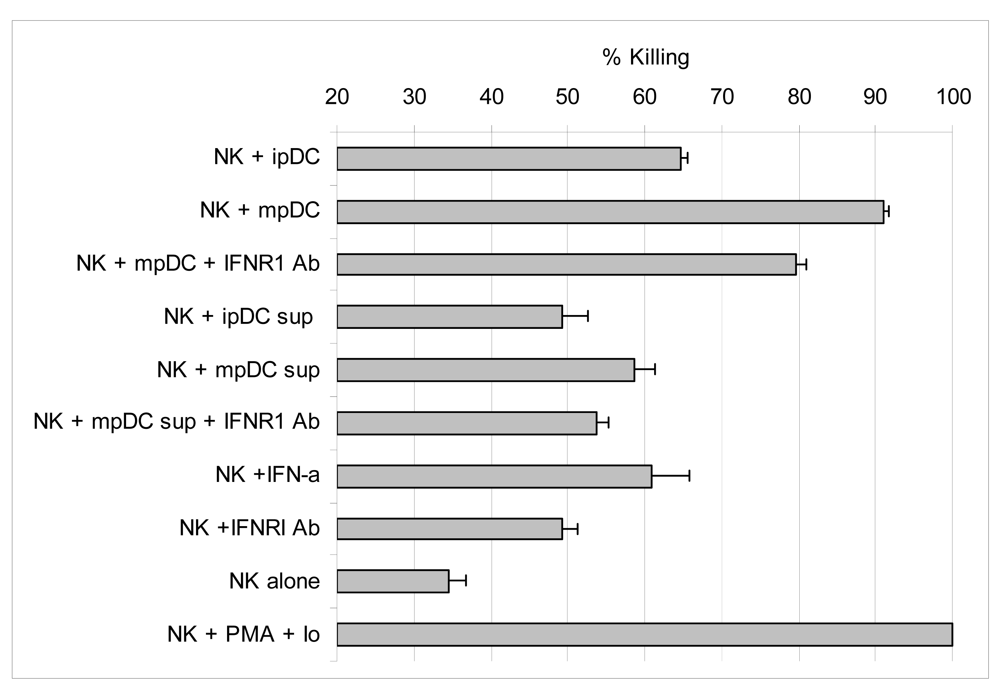
3.6 Activation of lytic function by NK cells is enhanced by IFNα, but mainly driven by direct contact with pDC
4. Discussion
Acknowledgments
References and Notes
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 767–811. [Google Scholar]
- Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 1989, 187–376. [Google Scholar]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 6673, 245–252. [Google Scholar]
- Rissoan, M.C.; Soumelis, V.; Kadowaki, N.; Grouard, G.; Briere, F.; de Waal, M.R.; Liu, Y.J. Reciprocal control of T helper cell and dendritic cell differentiation. Science 1999, 5405, 1183–1186. [Google Scholar]
- Liu, Y.J.; Kanzler, H.; Soumelis, V.; Gilliet, M. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2001, 7, 585–589. [Google Scholar]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999, 5421, 1835–1837. [Google Scholar]
- Krug, A.; Rothenfusser, S.; Hornung, V.; Jahrsdorfer, B.; Blackwell, S.; Ballas, Z.K.; Endres, S.; Krieg, A.M.; Hartmann, G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol. 2001, 7, 2154–2163. [Google Scholar]
- Grouard, G.; Rissoan, M.C.; Filgueira, L.; Durand, I.; Banchereau, J.; Liu, Y.J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997, 6, 1101–1111. [Google Scholar]
- Fonteneau, J.F.; Gilliet, M.; Larsson, M.; Dasilva, I.; Munz, C.; Liu, Y.J.; Bhardwaj, N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood 2003, 9, 3520–3526. [Google Scholar]
- Dzionek, A.; Fuchs, A.; Schmidt, P.; Cremer, S.; Zysk, M.; Miltenyi, S.; Buck, D.W.; Schmitz, J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000, 11, 6037–6046. [Google Scholar]
- Talmadge, J.E.; Meyers, K.M.; Prieur, D.J.; Starkey, J.R. Role of NK cells in tumour growth and metastasis in beige mice. Nature 1980, 5757, 622–624. [Google Scholar]
- Karre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986, 6055, 675–678. [Google Scholar]
- Lanier, L.L. Follow the leader: NK cell receptors for classical and nonclassical MHC class I. Cell 1998, 6, 705–707. [Google Scholar]
- Ljunggren, H.G.; Karre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 7, 237–244. [Google Scholar]
- Ferlazzo, G.; Tsang, M.L.; Moretta, L.; Melioli, G.; Steinman, R.M.; Munz, C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 2002, 3, 343–351. [Google Scholar]
- Fernandez, N.C.; Lozier, A.; Flament, C.; Ricciardi-Castagnoli, P.; Bellet, D.; Suter, M.; Perricaudet, M.; Tursz, T.; Maraskovsky, E.; Zitvogel, L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 1999, 4, 405–411. [Google Scholar]
- Piccioli, D.; Sbrana, S.; Melandri, E.; Valiante, N.M. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 2002, 3, 335–341. [Google Scholar]
- Wilson, J.L.; Heffler, L.C.; Charo, J.; Scheynius, A.; Bejarano, M.T.; Ljunggren, H.G. Targeting of human dendritic cells by autologous NK cells. J. Immunol. 1999, 12, 6365–6370. [Google Scholar]
- Chehimi, J.; Starr, S.E.; Kawashima, H.; Miller, D.S.; Trinchieri, G.; Perussia, B.; Bandyopadhyay, S. Dendritic cells and IFN-alpha-producing cells are two functionally distinct non-B, non-monocytic HLA-DR+ cell subsets in human peripheral blood. Immunology 1989, 4, 486–490. [Google Scholar]
- Gerosa, F.; Gobbi, A.; Zorzi, P.; Burg, S.; Briere, F.; Carra, G.; Trinchieri, G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J. Immunol. 2005, 2, 727–734. [Google Scholar]
- Romagnani, C.; Della, C.M.; Kohler, S.; Moewes, B.; Radbruch, A.; Moretta, L.; Moretta, A.; Thiel, A. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur. J. Immunol. 2005, 8, 2452–2458. [Google Scholar]
- Bauer, M.; Redecke, V.; Ellwart, J.W.; Scherer, B.; Kremer, J.P.; Wagner, H.; Lipford, G.B. Bacterial CpG-DNA triggers activation and maturation of human CD11c-, CD123+ dendritic cells. J. Immunol. 2001, 8, 5000–5007. [Google Scholar]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 1-2, 15–22. [Google Scholar]
- Caligiuri, M.A.; Zmuidzinas, A.; Manley, T.J.; Levine, H.; Smith, K.A.; Ritz, J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J. Exp. Med. 1990, 5, 1509–1526. [Google Scholar]
- Caligiuri, M.A.; Murray, C.; Robertson, M.J.; Wang, E.; Cochran, K.; Cameron, C.; Schow, P.; Ross, M.E.; Klumpp, T.R.; Soiffer, R.J. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J. Clin. Invest 1993, 1, 123–132. [Google Scholar]
- Mandelboim, O.; Lieberman, N.; Lev, M.; Paul, L.; Arnon, T.I.; Bushkin, Y.; Davis, D.M.; Strominger, J.L.; Yewdell, J.W.; Porgador, A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 2001, 6823, 1055–1060. [Google Scholar]
- Barao, I.; Hudig, D.; Ascensao, J.L. IL-15-mediated induction of LFA-1 is a late step required for cytotoxic differentiation of human NK cells from CD34+Lin- bone marrow cells. J. Immunol. 2003, 2, 683–690. [Google Scholar]
- Robertson, M.J.; Caligiuri, M.A.; Manley, T.J.; Levine, H.; Ritz, J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J. Immunol. 1990, 10, 3194–3201. [Google Scholar]
- D'Orazio, J.A.; Stein-Streilein, J. Human natural killer (NK) cells present staphylococcal enterotoxin B (SEB) to T lymphocytes. Clin. Exp. Immunol. 1996, 2, 366–373. [Google Scholar]
- Hanna, J.; Gonen-Gross, T.; Fitchett, J.; Rowe, T.; Daniels, M.; Arnon, T.I.; Gazit, R.; Joseph, A.; Schjetne, K.W.; Steinle, A.; Porgador, A.; Mevorach, D.; Goldman-Wohl, D.; Yagel, S.; LaBarre, M.J.; Buckner, J.H.; Mandelboim, O. Novel APC-like properties of human NK cells directly regulate T cell activation. J. Clin. Invest 2004, 11, 1612–1623. [Google Scholar]
- Cella, M.; Jarrossay, D.; Facchetti, F.; Alebardi, O.; Nakajima, H.; Lanzavecchia, A.; Colonna, M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999, 8, 919–923. [Google Scholar]
- Trinchieri, G.; Valiante, N. Receptors for the Fc fragment of IgG on natural killer cells. Nat. Immun. 1993, 4-5, 218–234. [Google Scholar]
- Biron, C.A. Activation and function of natural killer cell responses during viral infections. Curr.Opin.Immunol. 1997, 1, 24–34. [Google Scholar]
- Biron, C.A. Interferons alpha and beta as immune regulators--a new look. Immunity 2001, 6, 661–664. [Google Scholar]
- Santoli, D.; Trinchieri, G.; Koprowski, H. Cell-mediated cytotoxicity against virus-infected target cells in humans. II. Interferon induction and activation of natural killer cells. J. Immunol. 1978, 2, 532–538. [Google Scholar]
- Xiang, J.; Huang, H.; Liu, Y. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J. Immunol. 2005, 12, 7497–7505. [Google Scholar]
- Hwang, I.; Huang, J.F.; Kishimoto, H.; Brunmark, A.; Peterson, P.A.; Jackson, M.R.; Surh, C.D.; Cai, Z.; Sprent, J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J. Exp. Med. 2000, 7, 1137–1148. [Google Scholar]
- Ruby, J.; Ramshaw, I. The antiviral activity of immune CD8+ T cells is dependent on interferon-gamma. Lymphokine Cytokine Res. 1991, 5, 353–358. [Google Scholar]
- Young, H.A.; Hardy, K.J. Role of interferon-gamma in immune cell regulation. J. Leukoc. Biol. 1995, 4, 373–381. [Google Scholar]
- Nguyen, K.B.; Cousens, L.P.; Doughty, L.A.; Pien, G.C.; Durbin, J.E.; Biron, C.A. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat. Immunol. 2000, 1, 70–76. [Google Scholar]
- Orange, J.S.; Biron, C.A. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J. Immunol. 1996, 3, 1138–1142. [Google Scholar]
- Trinchieri, G.; Santoli, D.; Koprowski, H. Spontaneous cell-mediated cytotoxicity in humans: role of interferon and immunoglobulins. J. Immunol. 1978, 6, 1849–1855. [Google Scholar]
- Platsoucas, C.D.; Fox, F.E.; Oleszak, E.; Fong, K.; Nanno, M.; Ioannides, C.G.; Trotta, P.P. Regulation of natural killer cytotoxicity by recombinant alpha interferons. Augmentation by IFN-alpha 7, an interferon similar to IFN-alpha. J. Anticancer Res. 1989, 4, 849–858. [Google Scholar]
- Kohrgruber, N.; Halanek, N.; Groger, M.; Winter, D.; Rappersberger, K.; Schmitt-Egenolf, M.; Stingl, G.; Maurer, D. Survival, maturation, and function of CD11c- and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J. Immunol. 1999, 6, 3250–3259. [Google Scholar]
- Jinushi, M.; Takehara, T.; Kanto, T.; Tatsumi, T.; Groh, V.; Spies, T.; Miyagi, T.; Suzuki, T.; Sasaki, Y.; Hayashi, N. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J. Immunol. 2003, 3, 1249–1256. [Google Scholar]
- Masucci, M.G.; Masucci, G.; Klein, E.; Berthold, W. Target selectivity of interferon-induced human killer lymphocytes related to their Fc receptor expression. Proc. Natl. Acad.Sci. U.S.A. 1980, 6, 3620–3624. [Google Scholar]
- Gisslinger, H.; Kurzrock, R.; Gisslinger, B.; Jiang, S.; Li, S.; Virgolini, I.; Woloszczuk, W.; Andreeff, M.; Talpaz, M. Autocrine cell suicide in a Burkitt lymphoma cell line (Daudi) induced by interferon alpha: involvement of tumor necrosis factor as ligand for the CD95 receptor. Blood 2001, 9, 2791–2797. [Google Scholar]
- Oshima, K.; Yanase, N.; Ibukiyama, C.; Yamashina, A.; Kayagaki, N.; Yagita, H.; Mizuguchi, J. Involvement of TRAIL/TRAIL-R interaction in IFN-alpha-induced apoptosis of Daudi B lymphoma cells. Cytokine 2001, 4, 193–201. [Google Scholar]
- Grebenova, D.; Kuzelova, K.; Fuchs, O.; Halada, P.; Havlicek, V.; Marinov, I.; Hrkal, Z. Interferon-alpha suppresses proliferation of chronic myelogenous leukemia cells K562 by extending cell cycle S-phase without inducing apoptosis. Blood Cells Mol. Dis. 2004, 1, 262–269. [Google Scholar]
- Feldman, M.; Howell, D.; Fitzgerald-Bocarsly, P. Interferon-alpha-dependent and -independent participation of accessory cells in natural killer cell-mediated lysis of HSV-1-infected fibroblasts. J. Leukoc. Biol. 1992, 5, 473–482. [Google Scholar]
- Hanabuchi, S.; Watanabe, N.; Wang, Y.H.; Wang, Y.H.; Ito, T.; Shaw, J.; Cao, W.; Qin, F.X.; Liu, Y.J. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL). Blood 2006, 9, 3617–3623. [Google Scholar]



© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Benlahrech, A.; Donaghy, H.; Rozis, G.; Goodier, M.; Klavinskis, L.; Gotch, F.; Patterson, S. Human NK Cell Up-regulation of CD69, HLA-DR, Interferon γ Secretion and Cytotoxic Activity by Plasmacytoid Dendritic Cells is Regulated through Overlapping but Different Pathways. Sensors 2009, 9, 386-403. https://doi.org/10.3390/s90100386
Benlahrech A, Donaghy H, Rozis G, Goodier M, Klavinskis L, Gotch F, Patterson S. Human NK Cell Up-regulation of CD69, HLA-DR, Interferon γ Secretion and Cytotoxic Activity by Plasmacytoid Dendritic Cells is Regulated through Overlapping but Different Pathways. Sensors. 2009; 9(1):386-403. https://doi.org/10.3390/s90100386
Chicago/Turabian StyleBenlahrech, Adel, Heather Donaghy, George Rozis, Martin Goodier, Linda Klavinskis, Frances Gotch, and Steven Patterson. 2009. "Human NK Cell Up-regulation of CD69, HLA-DR, Interferon γ Secretion and Cytotoxic Activity by Plasmacytoid Dendritic Cells is Regulated through Overlapping but Different Pathways" Sensors 9, no. 1: 386-403. https://doi.org/10.3390/s90100386



