Fabrication of Poly(p-Phenylene)/Zeolite Composites and Their Responses Towards Ammonia
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Poly(p-phenylene) Synthesis
2.3. Poly(p-phenylene) Doping
2.4. Preparation of the Zeolitic Materials
2.5. Composite Preparation
2.6. Characterization
2.7. Electrical Conductivity and Gas Measurements
3. Results and Discussion
3.1. Characterization of Poly(p-phenylene)
3.2. Characterization of ZSM-5 and Composites
3.3. Electrical Conductivity in Air and N2
3.4. Electrical Conductivity Response to CO and H2
3.5. dPPP and Electrical Conductivity Sensitivity to NH3: Effect of NH3 Concentration
3.6. dPPP/ZSM-5(23) Composites and Electrical Conductivity Response to NH3: Effect of Zeolite Content
3.7. dPPP/ZSM-5(23) Composites and Electrical Conductivity Response to NH3: Effect of Cation Type
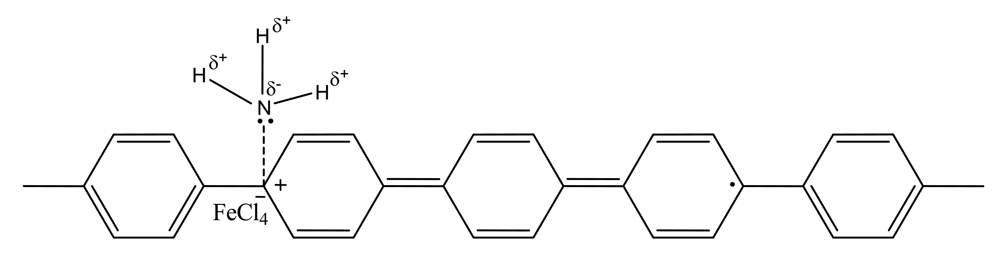
3.8. FTIR Investigations of Interactions of Adsorbed NH3
4. Conclusions
Acknowledgments
References and Notes
- Kaden, H.; Jahn, H.; Berthold, M. Study of the glass/polypyrrole interface in an all-solid-state pH sensor. Solid State Ionics 2004, 169, 129–133. [Google Scholar]
- Migdilski, J.; Blaz, T.; Lewenstam, A. Conducting polymer-based ion-selective electrodes. Anal. Chem. Acta 1996, 322, 141–149. [Google Scholar]
- Nohria, R.; Khillan, R.K.; Su, Y.; Dikshit, R.; Lvov, Y.; Varahramyan, K. Humidity sensor based on ultrathin polyaniline film deposited using layer-by-layer nano-assembly. Sens. Actuat. B-Chem. 2006, 114, 218–222. [Google Scholar]
- Grennan, K.; Killard, A.J.; Hanson, C.J.; Cafolla, A.A.; Smyth, M.R. Optimisation and characterisation of biosensors based on polyaniline. Talanta 2006, 68, 1591–1600. [Google Scholar]
- Persaud, K.C. Polymer in chemical sensing. Materials Today 2005, 8, 38–44. [Google Scholar]
- Watcharaphalakorn, S.; Ruangchuay, L.; Chotpattananont, D.; Srivat, A.; Schwank, J. Polyaniline/polyimide blends as gas sensors and electrical conductivity response to CO–N2 mixtures. Polym. Int. 2005, 54, 1126–1133. [Google Scholar]
- Ma, X.; Li, G.; Xu, H.; Wang, M.; Chen, H. Preparation of polythiophene composite film by in situ polymerization at room temperature and its gas response studies. Thin Solid Films 2006, 515, 2700–2704. [Google Scholar]
- Ruangchuay, L.; Sirivat, A.; Schwank, J. Polypyrrole/poly(methylmethacrylate) blend as selective sensor for acetone in lacquer. Talanta 2003, 60, 25–30. [Google Scholar]
- Vamstrom, C.; Ivaska, A. An electrochemical study of poly(paraphenylene) in conductive polymer bilayer electrodes. J. Electroanal. Chem. 1997, 421, 67–78. [Google Scholar]
- Blackwood, D.; Josowicz, M. Work function and spectroscopic studies of interaction between conducting polymers and organic vapors. J. Phys. Chem. 1991, 95, 493–502. [Google Scholar]
- Yoshino, K.; Gu, H.B. Effect of ammonium gas on electrical property of conductive polymers. J. Appl. Phys. 1986, 25, 1064–1068. [Google Scholar]
- Collins, G.E.; Buckley, L.J. Conductive polymer coated fabrics for chemical sensing. Synth. Metal. 1996, 78, 93–101. [Google Scholar]
- Bhat, N.V.; Gadre, A.P.; Bambole, V.A. Investigation of Electropolymerized polypyrrole composite film: characterization and application to gas sensors. J. Appl. Polym. Sci. 2003, 88, 22–29. [Google Scholar]
- Lähdesmäki, I.; Lewenstam, A.; Ivaska, A. A polypyrrole-based amperometric ammonia sensor. Talanta 1996, 43, 125–134. [Google Scholar]
- Nicolas, D.D.; Poncin, E.F. Polyaniline as a new sensitive layer for gas sensors. Anal. Chim. Acta 2003, 475, 1–15. [Google Scholar]
- Kukla, A.L.; Shirshov, Y.M.; Piletsky, S.A. Ammonia sensors based on sensitive polyaniline films. Sens. Actuat. B 1996, 37, 135–140. [Google Scholar]
- Singh, V.; Mohan, S.; Singh, G.; Pandey, P.C.; Prakash, R. Synthesis and characterization of polyaniline-carboxylated PVC composites: application in development of ammonia sensor. Sens. Actuat. B 2008, 132, 99–106. [Google Scholar]
- Airoudj, A.; Debarnot, D.; Bêche, B.; Poncin-Epaillard, F. Development of an optical ammonia sensor based on polyaniline/epoxy resin (SU-8) composite. Talanta 2008, 77, 1590–1596. [Google Scholar]
- Soontornworajit, B.; Wannatong, L.; Hiamtup, P.; Niamlang, S.; Chotpattananont, D.; Sirivat, A.; Schwank, J. Induced interaction between polypyrrole and SO2 via molecular sieve 13×. Mat. Sci. Eng. B-Solid 2007, 136, 78–86. [Google Scholar]
- Vilaseca, M.; Yague, C.; Coronas, J.; Santamaria, J. Development of QCM sensors modified by AlPO4-18 films. Sens. Actuat. B-Chem. 2006, 117, 143–150. [Google Scholar]
- Kovacic, P.; Kyriakis, A. Polymerization of benzene to p-polyphenyl by aluminum chloride-cupric chloride. J. Am. Chem. Soc. 1963, 85, 454–458. [Google Scholar]
- Kovacic, P.; Oziomek, J. p-Polyphenyl from benzene-Lewis acid catalyst-oxidant reaction scope and investigation of benzene-aluminum chloride-cupric chloride system. J. Org. Chem. 1964, 29, 100–104. [Google Scholar]
- Shiga, T.; Okada, A.; Kurauchi, T. Electroviscoelastic effect of polymer blends consisting of silicone elastomer and semiconducting polymer particles. Macromolecules 1993, 26, 6958–6963. [Google Scholar]
- Kuroda, Y.; Yoshikawa, Y. Characterization of specific N2-adsorption site existing on CuZSM-5 type zeolite: effect of ion-exchange level on adsorption properties. J. Phys. Chem. B 1999, 103, 2155–2164. [Google Scholar]
- Aeiyach, S.; Soubiran, P.; Lacaze, P. Influence of the temperature on the structure of poly(p-phenylene) (PPP) films formed by electropolymerization of benzene on platinum electrodes in sulphur dioxide medium. Polym. Commun. 1988, 29, 130–131. [Google Scholar]
- Yli-Lahti, P.; Stubb, H.; Isotalo, H.; Kuivalainen, P.; Kalervo, L. IR behaviour, conductivity and stability of FeCl3-doped polyparaphenylene (p-C6H4)x. Mol. Cryst. Liq. Cryst. 1985, 118, 305–308. [Google Scholar]
- Shacklette, L.W.; Eckhardt, H.; Chance, R.R.; Miller, G.G.; Ivory, D.M.; Baughman, R.H. Solid-state synthesis of highly conducting polyparaphenylene from crystalline oligomers. J. Chem. Phys. 1980, 73, 4098–4102. [Google Scholar]
- Yaniger, S.I.; Rose, D.J.; McKenna, W.P.; Eyring, E.M. Photoacoustic infrared spectroscopy of doped and undoped poly(p-phenylene). Macromolecules 1984, 17, 2579–2583. [Google Scholar]
- Oguma, J.; Akagi, K; Shirakawa, H. Synthesis and properties of liquid crystalline poly(p-phenylene) and poly(p-phenylenevinylene) derivatives. Synth. Metals 1999, 101, 86–103. [Google Scholar]
- Marvel, C.S.; Hartzell, G.E. Preparation and aromatization of poly-1,3-cyclohexadiene. J. Am. Chem. Soc. 1959, 81, 448–452. [Google Scholar]
- Kumar, R.; Cheng, W.C.; Rajagopalan, K.; Peters, A.W.; Basu, P. The Effect of Exchange Cations on Acidity, Activity, and Selectivity of Faujasite Cracking Catalysts. J. Catal. 1993, 143, 594–600. [Google Scholar]
- Pron, A.; Kulszewicz, I.; Billaud, D.; Pryluski, J. Organic metals reaction of FeCl3 with Polyacetylene, (CH)x, and poly-(p-phenylene), (p-C6H4)x. J. Chem. Soc. Chem. Commun. 1981, 15, 783–784. [Google Scholar]
- Chuapradit, C.; Wannatong, L.R.; Chotpattananont, D.; Sirivat, A.; Schwank, J. Polyaniline/zeolite LTA composites and electrical conductivity. Polymer 2005, 46, 947–953. [Google Scholar]
- Densakulprasert, N.; Wannatong, L.; Chotpattananont, D.; Hiamtup, P.; Sirivat, A.; Schwank, J. Electrical conductivity of polyaniline/zeolite composites and synergetic interaction with CO. Mat. Sci. Eng. B-Solid 2005, 117, 276–282. [Google Scholar]
- Yamamoto, K.; Gu, H.B. Effect of Ammonium Gas on Electrical Property of Conductive Polymers. Jpn. J. Appl. Phys. 1986, 25, 1064–1068. [Google Scholar]
- Kosacki, I.; Anderson, H.U. Nanostructured oxide thin films for gas sensors. Sens. Actuat. B-Chem. 1998, 48, 263–269. [Google Scholar]
- Shabnam, V.S.; Kaner, R.B.; Weiller, B.H. Hydrogen sensors based on conductivity changes in polyaniline nanofibers. J. Phys. Chem. B 2006, 110, 22266–22270. [Google Scholar]
- Bredas, J.R. Theoritical studies of charged defect states in doped polyacetylene and polyparaphenylene. Mol. Cryst. Liq. Cryst. 1981, 77, 319–332. [Google Scholar]
- Bradas, J.R. Comparative theoretical study of the doping of conjugated polymer: polarons in polyacetylene and polyparaphenylene. Phys. Rev. 1982, 26, 5843–5854. [Google Scholar]
- Yin, F.; Blumenfeld, L.; Gruver, V.; Fripait, J.J. NH3 as a probe molecule for NMR and IR study of zeolite catalyst acidity. J. Phys. Chem. B 1997, 101, 1824–1830. [Google Scholar]










| Cation | [Al3+] (mmol/g zeolite) | [Cation] (mmol/g zeolite) | % Exchange |
|---|---|---|---|
| Na+ | 1.0978 | 1.0674 | 97.23 |
| K+ | 1.0978 | 1.0576 | 96.34 |
| Zeolite | BET surface area (cm2/g) | Pore volume (cm3/g) |
|---|---|---|
| NH4ZSM-5(23) | 290.1 ± 0.85 | 0.1819 ± 0.0031 |
| HZSM-5(23) | 332.6 ± 6.51 | 0.2075 ± 0.0006 |
| NaZSM-5(23) | 283.1 ± 8.63 | 0.1759 ± 0.0112 |
| KZSM-5(23) | 273.3 ± 1.34 | 0.1663 ± 0.0074 |
| Samples | ti (min) | tre (min) | σ (S/cm) | Δσ (S/cm) | Δσ/ΔσN2 | ||
|---|---|---|---|---|---|---|---|
| Air | N2 | CO | |||||
| dPPP | 182 | 17 | (4.37 ± 0.13) × 10−1 | (7.41 ± 0.01) × 10−2 | (7.55 ± 0.02) × 10−2 | (1.37 ± 0.25) × 10−3 | (2.08 ± 0.19) × 10−2 |
| Samples | ti (min) | tre (min) | σ (S/cm) | Δσ (S/cm) | Δσ/ΔσN2 | ||
| Air | N2 | H2 | |||||
| dPPP | 133 | 10 | (7.56 ± 0.21) × 10−2 | (6.00 ± 0.01) × 10−2 | (6.01 ± 0.01) × 10−2 | (1.66 ± 2.27) × 10−4 | (5.14 ± 2.62) × 10−3 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Phumman, P.; Niamlang, S.; Sirivat, A. Fabrication of Poly(p-Phenylene)/Zeolite Composites and Their Responses Towards Ammonia. Sensors 2009, 9, 8031-8046. https://doi.org/10.3390/s91008031
Phumman P, Niamlang S, Sirivat A. Fabrication of Poly(p-Phenylene)/Zeolite Composites and Their Responses Towards Ammonia. Sensors. 2009; 9(10):8031-8046. https://doi.org/10.3390/s91008031
Chicago/Turabian StylePhumman, Pimchanok, Sumonman Niamlang, and Anuvat Sirivat. 2009. "Fabrication of Poly(p-Phenylene)/Zeolite Composites and Their Responses Towards Ammonia" Sensors 9, no. 10: 8031-8046. https://doi.org/10.3390/s91008031




