Direct Electrochemistry of Horseradish Peroxidase-Gold Nanoparticles Conjugate
Abstract
:1. Introduction
2. Results and Discussion
2.1. UV-Visible spectroscopy studies
2.2. Fourier transform infrared spectroscopy studies of Au-NPs
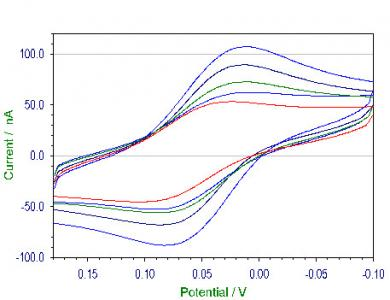
2.3. Electrochemical studies of HRP coupled to Au-NPs
2.3.1. Electrochemical studies of HRP coupled capped AuNPs
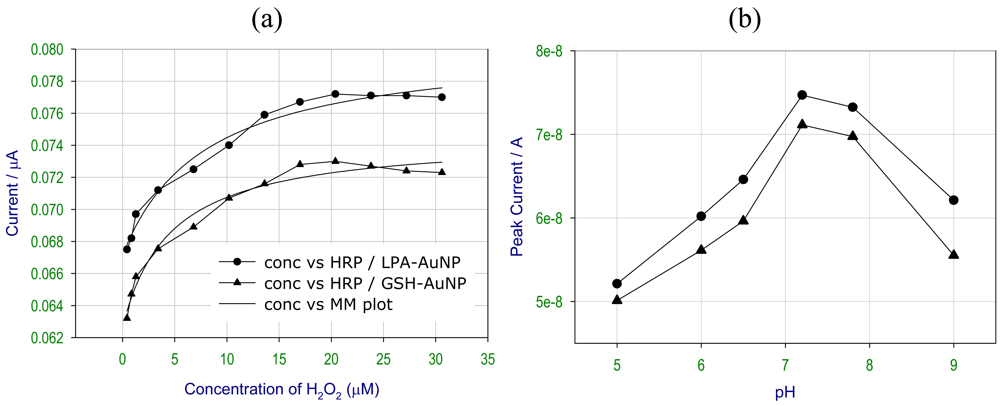
2.3.2. Electrochemical studies of HRP coupled to AuNPs at different concentrations of H2O2 and pH
2.4. Activity studies
3. Experimental Section
3.1. General
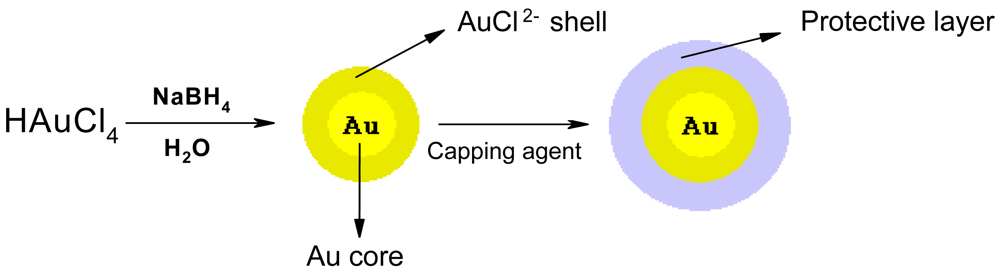
3.2. Preparation of gold nanoparticles (AuNP)
3.2.1. Preparation of glutathione capped AuNP
3.2.2. Preparation of lipoic acid capped AuNP
3.3. Preparation of AuNP –HRP conjugate
3.3.1. Activation of carboxylic groups in AuNP
3.3.2. Covalent linking of HRP with AuNP
4. Conclusions
Acknowledgments
References and Notes
- Storhoff, J.J.; Elghanian, R.; Mucic, R.C.; Mirkin, C.A.; Letsinger, R.L. One-Pot colorimetric differentiation of polynucleotides with singlebase imperfections using gold nanoparticle probes. J. Am. Chem. Soc. 1998, 120, 1959–1964. [Google Scholar]
- Hu, S.Q.; Xie, J.W.; Xu, Q.H.; Rong, K.T.; Shen, G.L.; Yu, R.Q. A label-free electrochemical immunosensor based on gold nanoparticles for detection of paraoxon. Talanta 2003, 61, 769–777. [Google Scholar]
- Kumar, S.; Aaron, J.; Sokolov, K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat. Protoc. 2008, 3, 314–320. [Google Scholar]
- Parak, W.J.; Gerion, D.; Pellegrino, T.; Zanchet, D.; Micheel, C.; Williams, S.C.; Boudreau, R.; Gros, M.AL.; Larabell, C.A.; Alivisatos, A.P. Biological applications of colloidal. Nanocrystals. Nanotechnology 2003, 14, R15–R27. [Google Scholar]
- Tkachenko, A.G.; Xie, H.; Coleman, D.; Glomm, W.; Ryan, J.; Anderson, M.F.; Franzen, S.; Feldheim, D.L. Multifunctional Gold nanoparticle-peptide complexes for nuclear targeting. J. Am. Chem. Soc. 2003, 125, 4700–4701. [Google Scholar]
- Burt, J.L.; Wing, C.G.; Yoshida, M.M.; Yacama, M.J. Noble-Metal nanoparticles directly conjugated to globular proteins. Langmuir 2004, 20, 11778–11783. [Google Scholar]
- Cai, H.; Xu, C.; He, P.; Fang, Y. Colloid Au-enhanced DNA immobilization for the electrochemical detection of sequence-specific DNA. J. Electroanal. Chem. 2001, 510, 78–85. [Google Scholar]
- Amirkin, C.; Letsinger, R.L.; Mucic, R.C.; Sterhoff, J.J. A DNA based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607–609. [Google Scholar]
- Castaneda, M.T.; Alegret, S.; Merkoci, A. Electrochemical sensing of DNA using gold nanoparticles. Electroanalysis 2007, 19, 743–753. [Google Scholar]
- Dequaire, M.; Degrand, C.; Limoges, B. An electrochemical metalloimmunoassay based on a colloidal gold label. Anal. Chem. 2000, 72, 5521–5528. [Google Scholar]
- Kumar, S.; Aaron, J.; Sokolov, K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat. protoc. 2008, 3, 314–320. [Google Scholar]
- Tang, H.; Chena, J.; Nie, L.; Kuang, Y.; Yao, S. A label-free electrochemical immunoassay for carcinoembryonic antigen (CEA) based on gold nanoparticles (AuNPs) and nonconductive polymer film. Biosens. Bioelectron. 2007, 22, 1061–1067. [Google Scholar]
- Authier, L.; Grossiord, C.; Brossier, P. Gold nanoparticle-based quantitative electrochemical detection of amplified human cytomegalovirus DNA using disposable microband electrodes. Anal. Chem. 2001, 73, 4450–4456. [Google Scholar]
- Li, X.; Yuan, R.; Chai, Y.; Zhang, Z.; Zhuo, Y.; Zhang, Y. Amperometric immunosensor based on toluidine blue/nano-Au through electrostatic interaction for determination of carcinoembryonic antigen. J. Biotechnol. 2006, 123, 356–366. [Google Scholar]
- Dua, D.; Liub, S.; Chena, J.; Jua, H.; Liana, H.; Lia, J. Colloidal gold nanoparticle modified carbon paste interface for studies of tumor cell adhesion and viability. Biomaterials 2005, 26, 6487–6495. [Google Scholar]
- Hua, S.Q.; Xie, J.W.; Xu, Q.H.; Rong, K.T.; Shen, G.L.; Yu, S.Q. A label-free electrochemical immunosensor based on gold nanoparticles for detection of paraoxon. Talanta 2003, 61, 769–777. [Google Scholar]
- Tkachenko, A.G.; Xie, H.; Coleman, D.; Glomm, W.; Ryan, J.; Anderson, M.F.; Franzen, S.; Feldheim, L. Multifunctional Gold Nanoparticle-Peptide Complexes for Nuclear Targeting. J. Am Chem. Soc. 2003, 125, 4700–4701. [Google Scholar]
- Wang, H.; Chen, Y.; Li, W.Y.; Liu, Y. Synthesis of oligo (ethylenediamino)-β-cyclodextrin modified gold nanoparticle as a DNA concentrator. Mol. Pharm. 2007, 4, 189–198. [Google Scholar]
- Yi, X.; Xian, J.H.; Yuan, C.H. Direct electrochemistry of horseradish peroxidase immobilized on a colloid/cysteamine-modified gold electrode. Anal. Biochem. 2000, 278, 22–28. [Google Scholar]
- Liu, S.; Ju, H. Electrocatalysis via direct electrochemistry of myoglobin immobilized on colloidal gold nanoparticles. Electroanalysis 2003, 15, 1488–1493. [Google Scholar]
- Ju, H.; Liu, S.; Ge, B.; Lisdat, F.; Scheller, F.W. Electrochemistry of cytochrome c immobilized on colloidal gold modified carbon paste electrodes and its electrocatalytic activity. Electroanalysis 2002, 14, 141–147. [Google Scholar]
- You, C.C.; De, M.; Han, G.; Rotello, V.N. Tunable inhibition and denaturation of α-Chymotrypsin with Amino Acid-Functionalized Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 12873–12881. [Google Scholar]
- Gole, A.; Dash, C.; Soman, C.; Sainkar, S.R.; Rao, M.; Sastry, M. On the preparation, characterization, and enzymatic activity of fungal protease gold colloid bioconjugates. Bioconjugate Chem. 2001, 12, 684–690. [Google Scholar]
- Frens, G. Controlled nucleation for the regulation of particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 30–32. [Google Scholar]
- Brust, M.; Fink, F.; Bethella, D.; Schiffrina, D.J.; Kiely, C. Synthesis and reactions of functionalised gold nanoparticles. J. Chem. Soc., Chem. Commun. 1995, 1655–1656. [Google Scholar]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, J.D.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc., Chem. Commun. 1994, 801–802. [Google Scholar]
- Selvakannan, P.R.; Mandal, S.; Phadtare, S.; Gole, A.; Pasricha, R.; Sastry, M. Water-dispersible tryptophan-protected gold nanoparticles prepared by the spontaneous reduction of aqueous chloroaurate ions by the amino acid. J. Colloid. Interface Sci. 2004, 269, 97–102. [Google Scholar]
- Subramaniam, C.; Tom, R.T.; Pradeep, T. On the formation of protected gold nanoparticles from AuCl4- by the reduction using aromatic amines. J. Nanopar. Res. 2005, 7, 209–217. [Google Scholar]
- Aslam, A.; Fu, L.; Su, M.; Vijayamohanan, K.; Dravid, V.P. Novel one-step synthesis of amine-stabilized aqueous colloidal gold nanoparticles. J. Mater. Chem. 2004, 14, 1795–1797. [Google Scholar]
- Wang, L.; Wei, G.; Sun, L.; Liu, Z. Self-assembly of cinnamic acid-capped gold nanoparticles. Nanotechnology 2006, 17, 2907–2912. [Google Scholar]
- Yonezawa1, T.; Nomura, T.; Kinoshita, T.; Koumoto, K. Preparation and characterization of polypeptide stabilized gold nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 1649–1650. [Google Scholar]
- Porta, F.; Speranza, G.; Krpeti, Z.; Santo, V.D.; Francescato, P. Gold nanoparticles capped by peptides. Mater. Sci. Eng., B 2007, 140, 187–194. [Google Scholar]
- Zheng, M.; Davidson, F.; Huang, X. Ethylene glycol monolayer protected nanoparticles for eliminating nonspecific binding with biological molecules. J. Am. Chem. Soc. 2003, 125, 7790–7791. [Google Scholar]
- Basu, S.; Pal, T. Glutathione-induced aggregation of gold nanoparticles: electromagnetic interactions in a closely packed assembly. J. Nanosci. Nanotechnol. 2007, 7, 1904. [Google Scholar]
- Yonezawa1, T.; Nomura, T.; Kinoshita, T.; Koumoto, K. Preparation and Characterization of Polypeptide-Stabilized Gold Nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 1649–1651. [Google Scholar]
- Ruzgas, T.; Csoregi, E.; Emnéus, J.; Gorton, L.; Varga, G.M. Peroxidase-modified electrodes: Fundamentals and application. Anal Chim Acta 1996, 330, 123–138. [Google Scholar]
- Razumas, V.J.; Gudavicius, A.V.; Kulys, J.J. Redox conversion of peroxidase on surface of-modified gold electrode. J. Electroanal Chem. 1983, 151, 311–315. [Google Scholar]
- Joensson, G. Reagentless hydrogen peroxide and glucose sensors based of peroxidase immobilized on graphite electrode. Electroanalysis 1991, 3, 741–750. [Google Scholar]
- Ruzgas, T.; Emnéus, J.; Lo, G.; Varga, G.J. Kinetic models of horseradish peroxidase action on a graphite electrode. J. Electroanal. Chem. 1995, 391, 41–49. [Google Scholar]
- Wollenberger, U.; Bogdanovskaya, V.; Bobsin, S.; Scheller, F.; Tarasevich, M. Enzyme electrode using bioelectrocatalytic reduction of Hydrogen peroxide. Anal. Letts. 1990, 23, 1795–1797. [Google Scholar]
- Gao, F.; Yuan, R.; Chai, Y.; Chen, S.; Cao, S.; Tang, M. Amperometric hydrogen peroxide biosensor based on the immobilization of HRP on nano-Au/Thi/poly (p-aminobenzene sulfonic acid)-modifiedglassy carbon electrode. J. Biochem. Biophys. Methods 2007, 70, 407–413. [Google Scholar]
- Yu, Z.; Qin, Z.H. Modified Electrode Based on Immobilizing Horseradish Peroxidase on nano-Gold with Choline Covalently Modified Glassy Carbon Electrode as a Base. Chin. J. Anal. Chem. 2008, 36, 604–610. [Google Scholar]
- Liu, Y.; Yuan, R.; Chai, Y.; Tang, D.; Dai, Y.; Zhong, X. Direct electrochemistry of horseradish peroxidase immobilized on gold colloid/cysteine/nafion-modified platinum disk electrode. Sens. Actuat., B 2006, 115, 109–115. [Google Scholar]
- Wang, Z.; Li, M; Su, P; Zhang, Y.; Shen, Y.; Han, D.; Niu, L. Direct electron transfer of horseradish peroxidase and its electrocatalysis based on carbon nanotube/thionine/gold composites. Electrochem. Commun. 2008, 10, 306–310. [Google Scholar]
- Luo, X.L.; Xu, J.J.; Zhang, Q.; Yang, G.J.; Chen, H.Y. Electrochemically deposited chitosan hydrogel for horseradish peroxidase immobilization through gold nanoparticles self-assembly. Biosens. Bioelectron. 2008, 21, 190–196. [Google Scholar]
- Di, J.; Shen, C.; Peng, S.; Tu, S.; Li, S. A one-step method to construct a third-generation biosensor based on horseradish peroxidase and gold nanoparticles embedded in silica sol–gel network on gold modified electrode. Anal. Chim. Acta 2005, 553, 196–200. [Google Scholar]
- Jia, J.; Wang, J.; Wu, A.; Cheng, G.; Li, Z.; Dong, S. A Method to Construct a Third-Generation Horseradish Peroxidase Biosensor: Self-Assembling Gold Nanoparticles to Three-Dimensional Sol–Gel Network. Anal. Chem. 2002, 74, 2217–2223. [Google Scholar]
- Xiang, C.; Zou, Y.; Sun, L.X.; Xu, F. Direct Electron Transfer of Horseradish Peroxidase and Its Biosensor Based on Gold Nanoparticles/Chitosan/ITO Modified Electrode. Anal. Letts. 2008, 41, 2224–2226. [Google Scholar]
- Liu, S.Q.; Ju, H.X. Renewable reagentless hydrogen peroxide sensor based on direct electron transfer of horseradish peroxidase immobilized on colloidal gold-modified electrode. Anal. Biochem. 2002, 307, 110–116. [Google Scholar]








© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ahirwal, G.K.; Mitra, C.K. Direct Electrochemistry of Horseradish Peroxidase-Gold Nanoparticles Conjugate. Sensors 2009, 9, 881-894. https://doi.org/10.3390/s90200881
Ahirwal GK, Mitra CK. Direct Electrochemistry of Horseradish Peroxidase-Gold Nanoparticles Conjugate. Sensors. 2009; 9(2):881-894. https://doi.org/10.3390/s90200881
Chicago/Turabian StyleAhirwal, Gautham Kumar, and Chanchal K. Mitra. 2009. "Direct Electrochemistry of Horseradish Peroxidase-Gold Nanoparticles Conjugate" Sensors 9, no. 2: 881-894. https://doi.org/10.3390/s90200881