Methodology and Significance of Microsensor-based Oxygen Mapping in Plant Seeds – an Overview
Abstract
:1. Introduction
2. Short Overview on Microsensors
- Affordable price,
- Measures oxygen in liquid as well as in the gas phase,
- Sensor signal independent of flow velocity,
- No time for polarization required, unlike the electrochemical electrode,
- No consumption of oxygen molecules while measuring, unlike the electrode that consumes oxygen molecules,
- No cross-sensitivity and no interference to carbon dioxide (CO2), hydrogen sulfide (H2S), ammonia (NH3), pH, and any ionic species like sulfide, sulfate or chloride. Oxygen microoptodes are only affected by gaseous sulfur dioxide (SO2) and gaseous chlorine (Cl2),
- Measurement range from 1 ppb up to 22.5 ppm dissolved oxygen
- Fast response times (t90 up to 1 s in the liquid and < 0.2 s in the gas phase).
3. Oxygen Mapping in Plant Seeds
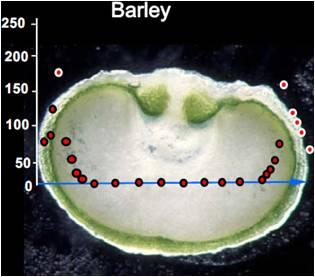
- The fruit (containing the intact seed) is fixed in a horizontal plane and, if necessary for the access of the microsensor, interfering material of the fruit is removed (e.g. a small window is cut into the pod wall of a leguminous species, while in maize, the husk is discarded).
- Correct positioning of the microsensor on the seed surface is aided by a microscope. In some cases, the sealing of the microsensor entry point is necessary to prevent the diffusion of oxygen into the seed via the micro-channels formed by the probe. Often this is achieved by the application of silicone grease.
- The microsensensor is driven, in a series of timed steps, into the seed using a micromanipulator. After each pulse, it is usual to pause for ∼ 10 s to allow equilibration and to obtain a local measurement.
- After measurement, the seed is dissected along the measurement transect to identify each of the structurally distinct zones of the seed (seed coat, vacuole, embryo, endosperm). This is necessary to relate measurements of oxygen concentration with each physiologically and functionally distinct portion of the seed.
4. Monitoring Environmental Effects on Steady-state Oxygen Concentrations within the Seed
5. The Establishment of Hypoxic Conditions during Seed Germination
6. Conclusions
Acknowledgments
References and Notes
- Boyle, S.A.; Yeung, E.C. Embryogeny of Phaseolus: Developmental pattern of lactate and alcohol dehydrogenases. Phytochemistry 1983, 22, 2413–2416. [Google Scholar]
- Wager, H.G. The effect of subjecting peas to air enriched with carbon dioxide I. Respiration and the metabolism of the major acids. J. Exp. Bot. 1974, 25, 338–351. [Google Scholar]
- Gale, J. Oxygen control of reproductive growth: possible mediation via dark respiration. J. Exp. Bot. 1974, 25, 987–989. [Google Scholar]
- Akita, S.; Tanaka, I. Studies on the mechanism of differences in photosynthesis among species III. Influence of low oxygen concentration on dry matter production and grain fertility of rice. CSSJ 1973, 42, 18–23. [Google Scholar]
- Quebedeaux, B.; Hardy, R.W.F. Reproductive growth and dry matter production of Glycine max (L.) Merr. In response to oxygen concentration. Plant Physiol. 1975, 55, 102–107. [Google Scholar]
- Borisjuk, L.; Rolletschek, H. The oxygen status of the developing seed. New Phytol. 2009, 182, 17–30. [Google Scholar]
- Revsbech, N.P.; J¢rgensen, B.B. Microelectrodes: Their use in microbial ecology. Marshall, K.C., Ed.; In Adv. Microb. Ecol.; 1986; Volume 9, pp. 293–352. [Google Scholar]
- KIimant, I.; Meyer, V.; Kühl, M. Fiber-optic oxygen microsensors, a new tool in aquatic biology. Limnol. Oceanogr. 1995, 40, 1159–1165. [Google Scholar]
- KIimant, I.; Holst, G.; Kühl, M. Oxygen microoptrodes and their application in aquatic environment. SPlE Proc. 1995. [Google Scholar]
- Holst, G.; Kühl, M.; KIimant, I. A novel measuring system for oxygen microoptodes based on a phase modulation technique. SPIE Proc. 1995. [Google Scholar]
- Volkmer, E.; Drosse, I.; Otto, S.; Stangelmayer, A.; Stengele, M.; Cherian Kallukalam, B.; Mutschler, W. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tiss. Eng. Part A. 2008, 14, 1331–1340. [Google Scholar]
- Bowling, D.J.F. Measurement of a gradient of oxygen partial pressure across the intact root. Planta 1973, 111, 323–28. [Google Scholar]
- Witty, J.F.; Skot, L.; Revsbech, N.P. Direct evidence for changes in the resistance of legume nodules to oxygen diffusion. J. Exp. Bot. 1987, 38, 1129–40. [Google Scholar]
- Armstrong, W.; Strange, M.E.; Cringle, S.; Beckett, P.M. Microelectrode and modelling study of oxygen distribution in roots. Ann. Bot. 1994, 74, 287–299. [Google Scholar]
- Armstrong, W.; Cousins, D.; Armstrong, J.; Turner, D.W.; Beckett, P.M. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Ann. Bot. 2000, 86, 687–703. [Google Scholar]
- Porterfield, D.M.; Kuang, A.; Smith, P.J.S.; Crispi, M.L.; Musgrave, M.E. Oxygen-depleted zones inside reproductive structures of Brassicaceae: implications for oxygen control of seed development. Can. J. Bot. 1999, 77, 1439–1446. [Google Scholar]
- Rolletschek, H.; Radchuk, R.; Klukas, C.; Schreiber, F.; Wobus, U.; Borisjuk, L. Evidence of a key role for photosynthetic oxygen release in oil storage in developing soybean seeds. New Phytol. 2005, 167, 777–786. [Google Scholar]
- Rolletschek, H.; Borisjuk, L.; Koschorreck, M.; Wobus, U.; Weber, H. Legume embryos develop in a hypoxic environment. J. Exp. Bot. 2002, 53, 1099–1107. [Google Scholar]
- Rolletschek, H.; Weber, H.; Borisjuk, L. Energy status and its control on embryogenesis of legumes. Embryo photosynthesis contributes to oxygen supply and is coupled to biosynthetic fluxes. Plant Physiol. 2003, 132, 1196–1206. [Google Scholar]
- Rolletschek, H.; Koch, K.; Wobus, U.; Borisjuk, L. Positional cues for the starch/lipid balance in maize kernels and resource partitioning to the embryo. Plant J. 2005, 42, 69–83. [Google Scholar]
- Rolletschek, H.; Weschke, W.; Weber, H.; Wobus, U.; Borisjuk, L. Energy state and its control on seed development: starch accumulation is associated with high ATP and steep oxygen gradients within barley grains. J. Exp. Bot. 2004, 55, 1351–1359. [Google Scholar]
- Rolletschek, H.; Borisjuk, L.; Sanchez-Garcia, A.; Romero, L.C.; Martinez-Rivas, J.M.; Mancha, M. Temperature-dependent endogenous oxygen concentration regulates microsomal oleate desaturase in developing sunflower seeds. J. Exp. Bot. 2007, 58, 3171–3181. [Google Scholar]
- VanDongen, J.T.; Roeb, G.W.; Dautzenberg, M.; Froehlich, A.; Vigeolas, H.; Minchin, P.E.H.; Geigenberger, P. Phloem import and storage metabolism are highly coordinated by the low oxygen concentrations within developing wheat seeds. Plant Physiol. 2004, 135, 1809–1821. [Google Scholar]
- Atkins, C.A.; Flinn, A.M. Carbon dioxide fixation in the carbon economy of developing seeds of Lupinus albus (L.). Plant Physiol. 1978, 62, 486–490. [Google Scholar]
- Eastmond, P.; Kolacna, L.; Rawsthorne, S. Photosynthesis by developing embryos of oilseed rape. J. Exp. Bot. 1996, 47, 1763–1769. [Google Scholar]
- Borisjuk, L.; Macherel, D.; Benamar, A.; Wobus, U.; Rolletschek, H. Low oxygen sensing and balancing in plant seeds – a role for nitric oxide. New Phytol. 2007, 176, 813–823. [Google Scholar]
- Dordas, C.; Hasinoff, B.B.; Igamberdiev, A.U.; Manaćh, N.; Rivoal, J.; Hill, R.D. Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J. 2003, 35, 763–770. [Google Scholar]
- Hagen, T.; Taylor, C.T.; Lam, F.; Moncada, S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on Hif1α. Science 2003, 302, 1975–1978. [Google Scholar]
- Moncada, S.; Erusalimsky, J.D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 2002, 3, 214–220. [Google Scholar]
- Yamasaki, H.; Shimoji, H.; Ohshiro, Y.; Sakihama, Y. Inhibitory effects of nitric oxide on oxidative phosphorylation in plant mitochondria. Nitric Oxide: Biol. Chem. 2001, 5, 261–270. [Google Scholar]
- Al-Ani, A.; Bruzau, F.; Raymond, P.; Saint-Ges, V.; Leblanc, J.M.; Pradet, A. Germination, respiration, and adenylate energy charge of seeds at various oxygen partial pressures. Plant Physiol. 1985, 79, 885–890. [Google Scholar]
- Corbineau, F.; Come, D. Control of seed germination and dormancy by the gaseous environment. In Seed Development and Germination; Kigel, J, Galili, G, Eds.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 397–424. [Google Scholar]
- Fait, A.; Angelovici, R.; Less, H; Ohad, I; Urbanczyk-Wochniak, E; Fernie, AR; Galili, G. Arabidopsis seed development and germination is associated with temporally distinct metabolic switches. Plant Physiol. 2006, 142, 839–854. [Google Scholar]
- Sreenivasulu, N.; Usadel, B.; Winter, A.; Radchuk, V.; Scholz, U.; Stein, N.; Weschke, W.; Strickert, M.; Close, T.J.; Stitt, M.; Graner, A.; Wobus, U. Barley grain maturation and germination: metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol. 2008, 146, 1738–1758. [Google Scholar]
- Chao, C.C.; Lin, T.P. Content of adenylate nucleotides and energy charge in the early stage of germination of orthodox and recalcitrant seeds. Bot. Bull. Acad. Sinica. 1996, 37, 229–237. [Google Scholar]
- Benamar, A.; Rolletschek, H.; Borisjuk, L.; Avelange-Macherel, M.H.; Curien, G.; Mostefai, H.A.; Andriantsitohaina, R.; Macherel, D. Nitrite-nitric oxide control of mitochondrial respiration at the frontier of anoxia. Biochim. Biophys. Acta. 2008, 1777, 1268–1275. [Google Scholar]
- Raymond, P.; Al-Ani, A.; Pradet, A. ATP production by respiration and fermentation, and energy charge during aerobiosis and anaerobiosis in twelve fatty and starchy germinating seeds. Plant Physiol. 1985, 79, 879–884. [Google Scholar]
- Borisjuk, L.; Nguyen, T.H.; Neuberger, T.; Rutten, T.; Tschiersch, H.; Claus, B.; Feussner, I.; Webb, A.G.; Jacob, P.; Weber, H.; Wobus, U.; Rolletschek, H. Gradients of lipid storage, photosynthesis and plastid differentiation in developing soybean seeds. New Phytol. 2005, 167, 761–776. [Google Scholar]



© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rolletschek, H.; Stangelmayer, A.; Borisjuk, L. Methodology and Significance of Microsensor-based Oxygen Mapping in Plant Seeds – an Overview. Sensors 2009, 9, 3218-3227. https://doi.org/10.3390/s90503218
Rolletschek H, Stangelmayer A, Borisjuk L. Methodology and Significance of Microsensor-based Oxygen Mapping in Plant Seeds – an Overview. Sensors. 2009; 9(5):3218-3227. https://doi.org/10.3390/s90503218
Chicago/Turabian StyleRolletschek, Hardy, Achim Stangelmayer, and Ljudmilla Borisjuk. 2009. "Methodology and Significance of Microsensor-based Oxygen Mapping in Plant Seeds – an Overview" Sensors 9, no. 5: 3218-3227. https://doi.org/10.3390/s90503218