A Novel Label-Free Optical Biosensor Using Synthetic Oligonucleotides from E. coli O157:H7: Elementary Sensitivity Tests
Abstract
:1. Introduction
2. Experimental
2.1. SiO2-TiO2 Thin Films Preparation
2.2. DNA Immobilization & Hybridization
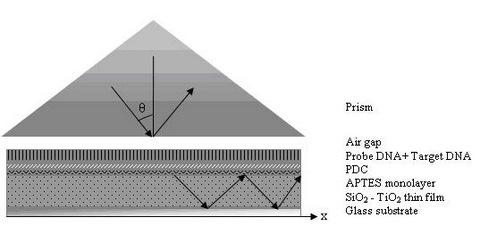
2.3. Prism Coupler Setup
3. Results and Discussion
4. Conclusions
Acknowledgments
References and Notes
- Lawrence, C.R.; Geddes, N.J. Handbook of Biosensors and Electronic Noses: Medicine, Food and the Environment.; Kress-Rogers, E., Ed.; CRC: New York, NY, 1997; Volume Chapter 7, p. 227. [Google Scholar]
- Rodriguez-Mozaz, S.; de Alda, M.J.L.; Marco, M.P.; Barceló, D. Biosensors for environmental monitoring. Talanta 2005, 65, 291–297. [Google Scholar]
- Brett, A.M.O. Biosensors and modern biospecific analytical techniques; Gorton, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume Chapter 4, p. 179. [Google Scholar]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar]
- Basic Description. http://www.metricon.com/basic.htm.
- Rong, G.; Najmaie, A.; Sipe, J.E.; Weiss, S.M. Nanoscale porous silicon waveguide for label-free DNA sensing. Biosens. Bioelectron. 2008, 23, 1572–1576. [Google Scholar]
- Li, F.; Chen, W.; Zhang, S. Development of DNA electrochemical biosensor based on covalent immobilization of probe DNA by direct coupling of sol–gel and self-assembly technologies. Biosens. Bioelectron. 2008, 24, 781–786. [Google Scholar]
- Łukowiak, A; Dylewicz, R.; Patela, S.; Stręk, W.; Maruszewski, K. Optical properties of SiO2–TiO2 thin film waveguides obtained by the sol–gel method and their applications for sensing purposes. Opt. Mat. 2005, 27, 1501–1505. [Google Scholar]
- Bahşi, Z.B.; Oral, A.Y. Effects of Mn and Cu doping on the microstructures and optical properties of sol–gel derived ZnO thin films. Opt. Mat. 2007, 29, 672–678. [Google Scholar]
- Leonard, P.; Hearty, S.; Brennan, J.; Dunne, L.; Quinn, J.; Chakraborty, T.; O'Kennedy, R. Advances in biosensors for detection of pathogens in food and water. Enzyme Microb. Technol. 2003, 32, 3–13. [Google Scholar]
- Almadidy, A.; Watterson, J.; Piunno, P.A.E.; Raha, S.; Faulds, I.V.; Horgen, P.A.; Castle, A.; Krull, U. Direct selective detection of genomic DNA from coliform using a fiber optic biosensor. Anal Chim Acta 2002, 461, 37–47. [Google Scholar]
- Dufour, A.P. Escherichia coli: The Fecal Coliform. In Bacterial indicators/Health Hazards Associated with Water, ASTM STP635.; Hoadley, A.W., Dutka, B.J., Eds.; American Society for Testing and Materials: Philadelphia, PA, USA, 1977; pp. 48–58. [Google Scholar]
- Drinking water contaminants. http://www.epa.gov/safewater/contaminants/ecoli.html.
- Simpson, J.M.; Lim, D.V. Rapid PCR confirmation of E.coli O157:H7 after evanescent wave fiber optic biosensor detection. Biosens. Bioelectron. 2005, 21, 881–887. [Google Scholar]
- Ulrich, R.; Torge, R. Measurement of Thin Film Parameters with a Prism Coupler. Appl. Opt. 1973, 12, 2901–2908. [Google Scholar]
- Wang, H.; Zhong, W.; Xu, P.; Du, Q. Polyimide/silica/titania nanohybrids via a novel non-hydrolytic sol–gel route. Compos. Part A-Appl. S. 2005, 36, 909–914. [Google Scholar]
- Tian, X.; Chen, Q.; Song, L.; Wang, Y.; Li, H. Formation of alkali resistant PDMS–TiO2–SiO2 hybrid coatings. Mater Lett. 2007, 61, 4432–4434. [Google Scholar]
- Song, C.F.; Lü, M.K.; Yang, P.; Xu, D.; Yuan, D.R. Structure and photoluminescence properties of sol–gel TiO2–SiO2 films. Thin Solid Film. 2002, 413, 155–159. [Google Scholar]
- Festag, G.; Steinbrück, A.; Wolff, A.; Csaki, A.; Möller, R.; Fritzsche, W. Optimization of gold nanoparticle-based DNA detection for microarrays. J. Fluoresc. 2005, 15, 161–170. [Google Scholar]
- Rong, G.; Saarinen, J.J.; Sipe, J.E.; Weiss, S.M. High sensitivity sensor based on porous silicon waveguide. Material Research Society Symposium Proceedings, San Francisco, USA; 2006. [Google Scholar]
- Rong, G.; Najmaie, A.; Sipe, J.E.; Weiss, S.M. Porous silicon waveguides for DNA detection. 3rd IEEE International Conference on Group IV Photonics, Ottawa, ON, Camada; 2006; pp. 13–15. [Google Scholar]








| HEPES Buffer | Probe DNA | Target DNA | |
|---|---|---|---|
| Mean refractive index values | 1.6928 | 1.6937 | 1.6949 |
| Standard deviation | 0.00009 | 0.00008 | 0.00012 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bahşi, Z.B.; Büyükaksoy, A.; Ölmezcan, S.M.; Şimşek, F.; Aslan, M.H.; Oral, A.Y. A Novel Label-Free Optical Biosensor Using Synthetic Oligonucleotides from E. coli O157:H7: Elementary Sensitivity Tests. Sensors 2009, 9, 4890-4900. https://doi.org/10.3390/s90604890
Bahşi ZB, Büyükaksoy A, Ölmezcan SM, Şimşek F, Aslan MH, Oral AY. A Novel Label-Free Optical Biosensor Using Synthetic Oligonucleotides from E. coli O157:H7: Elementary Sensitivity Tests. Sensors. 2009; 9(6):4890-4900. https://doi.org/10.3390/s90604890
Chicago/Turabian StyleBahşi, Zehra Banu, Aligül Büyükaksoy, Sinan Mert Ölmezcan, Fethi Şimşek, Muhammed Hasan Aslan, and Ahmet Yavuz Oral. 2009. "A Novel Label-Free Optical Biosensor Using Synthetic Oligonucleotides from E. coli O157:H7: Elementary Sensitivity Tests" Sensors 9, no. 6: 4890-4900. https://doi.org/10.3390/s90604890