Antioxidant Activity and Total Phenolic and Flavonoid Contents of Hieracium pilosella L. Extracts
Abstract
:1. Introduction
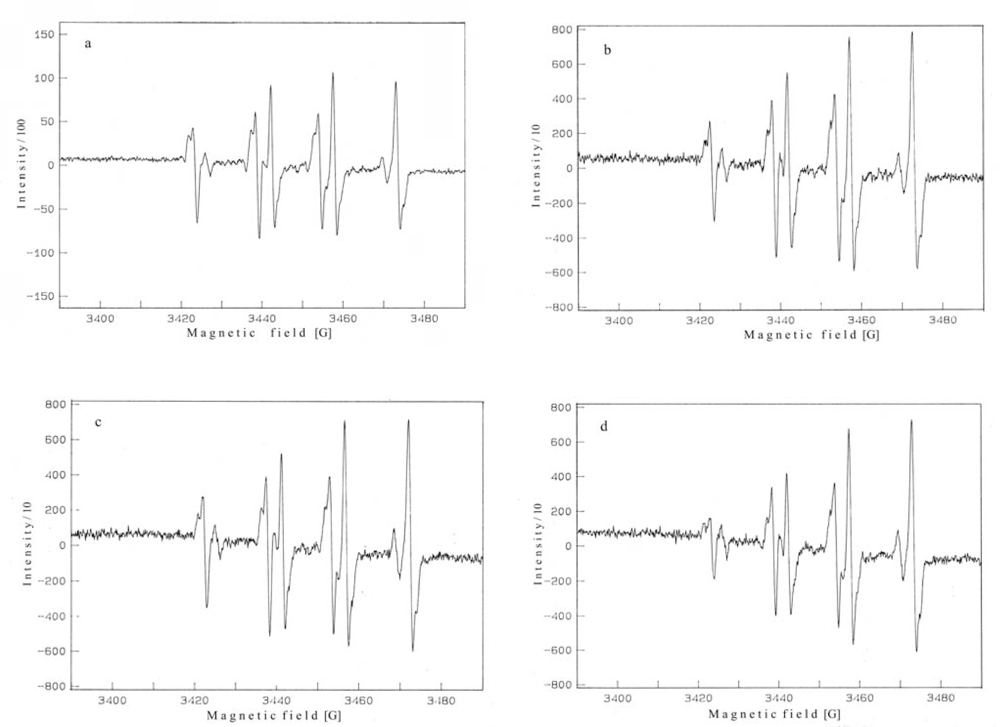
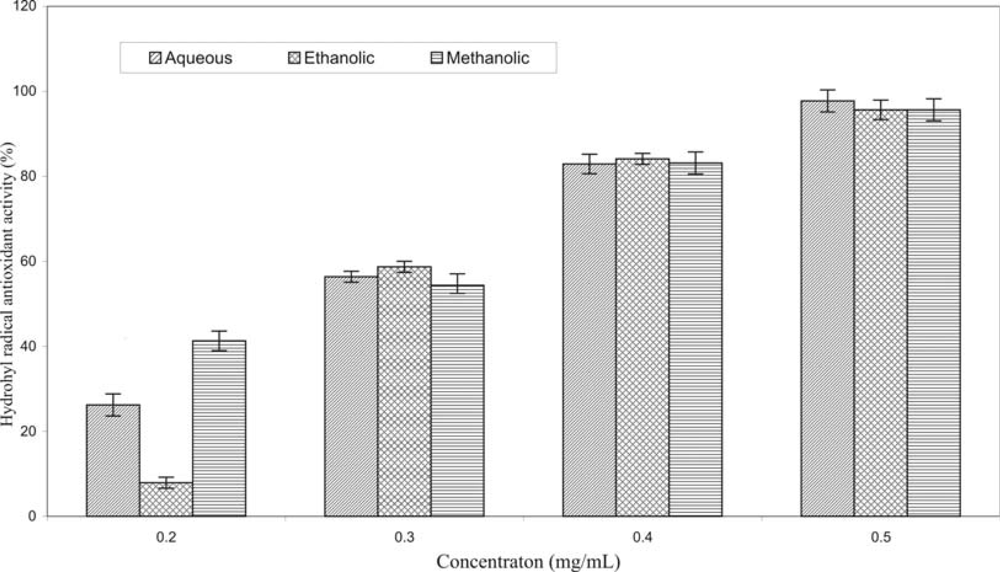
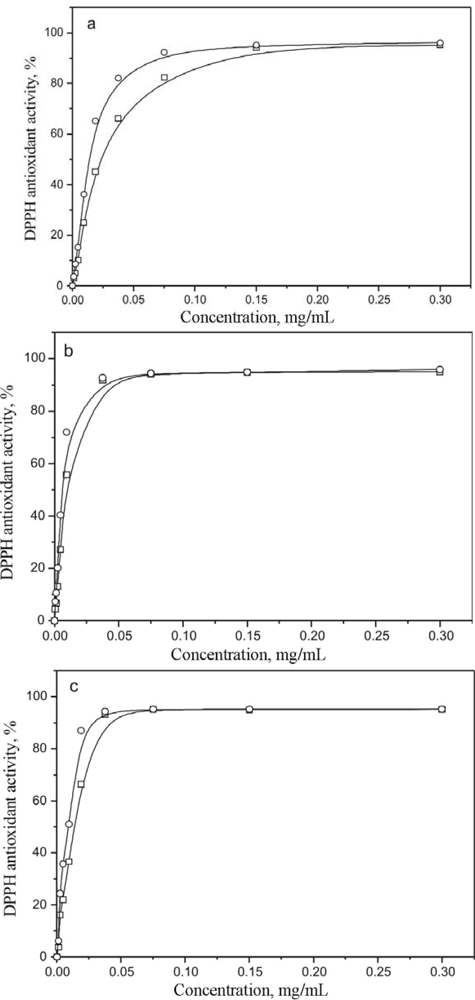
2. Results and Discussion
3. Experimental Section
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Extraction Method-Soxhlet Extraction
3.4. Determination of Plant Extract Yield
3.5. HPLC Analysis
3.6. DPPH• Assay
3.7. Hydroxyl Radical Assay
3.8. Determination of Total Phenolic Content
3.9. Determination of Total Flavonoid Content
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
References and Notes
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol 2006, 5, 1142–1145. [Google Scholar]
- Wong, S.P.; Leong, L.P.; Koh, J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food Chem 2006, 99, 775–783. [Google Scholar]
- Su, L.; Yin, J.-J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem 2007, 100, 990–997. [Google Scholar]
- Tepe., B.; Eminagaoglu, O.; Akpulat, H.A.; Aydin, E. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia verticillata (L.) subsp. verticillata and S. verticillata (L.) subsp. amasiaca (Freyn & Bornm.) Bornm. Food Chem 2007, 100, 985–989. [Google Scholar]
- Dillard, C.J.; German, J.B. Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric 2000, 80, 1744–1756. [Google Scholar]
- Turkoglu, A.; Duru, M.E.; Mercan, N.; Kivrak, I.; Gezer, K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem 2007, 101, 267–273. [Google Scholar]
- Canadanovic-Brunet, J.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T. Free-radical scavenging activity of wormwood (Artemisia absinthium L.) extracts. J. Sci. Food Agric 2005, 85, 265–272. [Google Scholar]
- Pietta, P.G. Flavonoids and antioxidants. J. Nat. Prod 2000, 63, 1035–1042. [Google Scholar]
- Marimuthu, P.; Wu, C.-L.; Chang, H.-T.; Chang, S.-T. Antioxidant activity of the ethanolic extract from the bark of Chamaecyparis obtusa var. formosana. J. Sci. Food Agric 2008, 88, 1400–1405. [Google Scholar]
- Ito, N.; Fukushina, S.; Tsuda, H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT and other antioxidants. CRC Crit. Rev. Toxicol 1985, 15, 109–115. [Google Scholar]
- Canadanovic-Brunet, J.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T.; Mandic, A.I.; Canadanovic, V.M. Antioxidant activities of different Teucrium montanum L. extracts. Int. J. Food Sci. Technol 2006, 41, 667–673. [Google Scholar]
- Djilas, S.M.; Canadanovic-Brunet, J.M.; Cetkovic, G.S.; Tumbas, V.T. Antioxidative activity of some herbs and species - review of ESR studies. In Magnetic resonance in Food Science; Belton, P.S., Gill, A.M., Webb, G.A., Rutledge, D., Eds.; RSC: Cambridge, UK, 2003. [Google Scholar]
- Nostro, A.; Germanò, M.P.; D’Angelo, V.; Marino, A.; Cannetelli, M.A. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microbiol 2000, 30, 379–384. [Google Scholar]
- Milic, B.L.J.; Djilas, S.M.; Canadanović-Brunet, J.M.; Sakac, M.B. Polyphenols in Plants. Faculty of Technology, University of Novi Sad, Novi Sad,. 2000; 277–309. [Google Scholar]
- Petrovic, S.D.; Gorunovic, M.S.; Wray, V.; Merfort, I. A taraxasterol derivate and compounds from Hieracium gymnocephalum. Phytochemistry 1999, 50, 293–296. [Google Scholar]
- Stanojevic, L.P.; Stankovic, M.Z.; Nikolic, V.D.; Nikolic, L.B. Anti-oxidative and antimicrobial activities of Hieracium pilosella L. extracts. J. Serb. Chem. Soc 2008, 73, 531–540. [Google Scholar]
- Randjelovic, N.; Jeremic, Z.; Stamenkovic, V. Healing Plants of Timok Region, Phytotherapy II; Young Explorer Organization and Cultural and Educational Community of Zajecar; Zajecar, 1995. [Google Scholar]
- Zidorn, C.; Schubert, B.; Stuppner, H. Altitudinal differences in the contents of phenolics in flowering heads of three members of the tribe Lactuceae (Asteraceae) occurring as introduced species in New Zealand. Biochem. Syst. Ecol 2005, 33, 855–872. [Google Scholar]
- Petrovovic, S.D.; Löscher, R.; Gorunovic, M.S.; Merfort, I. Flavonoid and phenolic acid petterns in seven Hieracium species. Biochem. System. Ecol 1999, 27, 651–656. [Google Scholar]
- Makepeace, W.; Dobson, E.T.; Scott, D. Interference phenomena due to mouse ear and king devil hawkweed. New. Zeal. J. Bot 1985, 23, 79–90. [Google Scholar]
- Konczak, I.; Okuno, S.; Yoshimoto, M.; Yamakawa, O. Caffeoylquinic acids generated in vitro in a high-anthocyanin-accumuleting sweet poteto cell line. J. Biomed. Biotechnol 2004, 5, 287–292. [Google Scholar]
- Kosar, M.; Dorman, D.; Baser, K.; Hiltunen, R. An improved HPLC post-column methodology for the identificetion of free radical scavenging phytochemicals in complex mixtures. Chromatographia 2004, 60, 635–638. [Google Scholar]
- Oboh, G.; Raddatz, H.; Henle, T. Antioxidant properties of polarand non-polar extracts of some tropical green leafy vegetables. J. Sci. Food. Agric 2008, 88, 2486–2492. [Google Scholar]
- Kampa, M.; Alexaki, V.I.; Notas, G.; Nifli, A.P.; Nistikaki, A.; Hetzoglou, A. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast. Cancer. Res 2004, 6, 63–74. [Google Scholar]
- Caccetta, R.A.A.; Croft, K.D.; Beilin, L.J.; Puddey, I.B. Ingestion of red wine significantly increases plasma phenolic acid concentrations but does not acutely affect ex vivo lipoprotein oxidizability. Am. J. Clin. Nutr 2000, 71, 67–74. [Google Scholar]
- Wen, A.M.; Delaquis, P.; Stanich, K.; Toivonen, P. Antilisterial activity of selected phenolic acids. Food Microbiol 2003, 20, 305–311. [Google Scholar]
- Proestos, C.; Boziaris, I.S.; Nychas, G.-J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem 2006, 95, 664–671. [Google Scholar]
- Chen, Y.; Yu, Q.J.; Li, X.; Luo, Y.; Liu, H. Extraction and HPLC characterization of chlorogenic acid from tobacco residuals. Sep. Sci. Technol 2007, 42, 3481–3492. [Google Scholar]
- Maisuthisakul, P.; Suttajit, M.; Pongsawatmanit, R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem 2007, 100, 1409–1418. [Google Scholar]
- Stanojevic, L.P.; Stankovic, M.Z.; Nikolic, L.B.; Nikolic, V.D. The influence of the operation conditions and the extraction techniques on the yield, kinetics and composition of ethanol extracts of Hieracium pilosella L. Chem. Ind. Chem. Eng. Quart 2007, 13, 199–204. [Google Scholar]
- Stanojevic, L.P.; Stankovic, M.Z.; Cakic, M.D.; Nikolic, V.D.; Nikolic, L.B.; Ristic, D.P. The effect of the operation conditions and the extraction techniques on the yield, kinetics and composition of methanol extracts of Hieracium pilosella L. Hem. Ind 2009, 63, 79–86. [Google Scholar]
- Aquino, R.; Morelli, S.; Tomaino, A.; Pellegrino, M.; Saija, A.; Grumetto, L.; Puglia, C.; Ventura, D.; Bonina, F.; Grumetto, L. Antioxidant and photoprotective activity of a crude extract of Culcitium reflexum H. B. K. Leaves and their major flavonoids. J. Ethnopharmacol 2002, 79, 183–191. [Google Scholar]
- Choi, W.C.; Kim, C.S.; Hwang, S.S.; Choi, K.B.; Ahn, J.H.; Lee, Y.M.; Park., H.S.; Kim, K.S.; Lee, Y.M. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant. Sci 2002, 163, 1161–1168. [Google Scholar]
- Lu, Li.-C.; Chen, Y.-W.C.; Chou, C.-C. Antibacterial and DPPH free radical-scavenging activities of the ethanol extract of propolis collected in Taiwan. J. Food Drug. Anal 2003, 11, 277–282. [Google Scholar]
- Sanchez-Moreno, C. Methods Used to Evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int 2002, 8, 121–137. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Meth. Enzymol 1999, 299, 152–178. [Google Scholar]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem 2007, 101, 140–147. [Google Scholar]
| Solvent | Total extract (g/100 g of dry plant material) |
|---|---|
| Water | 38.18 ± 1.13 |
| Ethanol (50% v/v) | 44.0 ± 1.03 |
| Methanol (80% v/v) | 42.33 ± 0 86 |
| Chlorogenic acid | Umbelliferone | Apigenin-7-O-glucoside | ||||
|---|---|---|---|---|---|---|
| Extract | Dry extract | Dry plant material | Dry extract | Dry plant material | Dry extract | Dry plant material |
| Aqueous | 52.30 ± 1.25 | 19.97 ± 0.85 | 1.69 ± 0.10 | 0.65 ± 0.06 | 0.21 ± 0.02 | 0.079 ± 0.08 |
| Ethanolic | 49.10 ± 1.20 | 21.60 ± 1.52 | 0.72 ± 0.15 | 0.31 ± 0.05 | 0.58 ± 0.03 | 0.250 ± 0.04 |
| Methanolic | 45.63 ± 1.10 | 19.20 ± 1.05 | 1.36 ± 0.2 | 0.58 ± 0.06 | 0.16 ± 0.04 | 0.068 ± 0.02 |
| Extract | EC50DPPH, mg/mL | Total phenolic content, mgGAE/g | Total flavonoids, mgRE/g | |
|---|---|---|---|---|
| Without incubation | 20 minutes incubation | |||
| Aqueous | 0.023 ± 4×10−4 | 0.011 ± 2×10−4 | 239.59 ± 2.03 | 79.13 ± 0.47 |
| Ethanolic | 0.011 ± 5×10−4 | 0.007 ± 10−4 | 244.16 ± 2.15 | 82.18 ± 0.53 |
| Methanolic | 0.014 ± 3×10−4 | 0.009 ± 10−4 | 243.98 ± 2.14 | 81.52 ± 0.24 |
| Compound | Chlorogenic acid | Apigenin-7-O-glucoside | Umbelliferone |
|---|---|---|---|
| Retention time, min | 2.07 | 4.41 | 4.99 |
| Concentration range, μg/mL | 1 – 500 | 0.15 – 15 | 4 – 670 |
| Calibration curve P[mAU]=q+r×c[mg/mL]* | q = 75.84 r = 30891.11 | q = 60.08 r = 79938.97 | q = 235.61 r = 153295.95 |
| Correlation coefficient | 0.9998 | 0.9997 | 0.9998 |
© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stanojević, L.; Stanković, M.; Nikolić, V.; Nikolić, L.; Ristić, D.; Čanadanovic-Brunet, J.; Tumbas, V. Antioxidant Activity and Total Phenolic and Flavonoid Contents of Hieracium pilosella L. Extracts. Sensors 2009, 9, 5702-5714. https://doi.org/10.3390/s90705702
Stanojević L, Stanković M, Nikolić V, Nikolić L, Ristić D, Čanadanovic-Brunet J, Tumbas V. Antioxidant Activity and Total Phenolic and Flavonoid Contents of Hieracium pilosella L. Extracts. Sensors. 2009; 9(7):5702-5714. https://doi.org/10.3390/s90705702
Chicago/Turabian StyleStanojević, Ljiljana, Mihajlo Stanković, Vesna Nikolić, Ljubiša Nikolić, Dušica Ristić, Jasna Čanadanovic-Brunet, and Vesna Tumbas. 2009. "Antioxidant Activity and Total Phenolic and Flavonoid Contents of Hieracium pilosella L. Extracts" Sensors 9, no. 7: 5702-5714. https://doi.org/10.3390/s90705702




