Fabrication of a Multi-Walled Nanotube (MWNT) Ionic Liquid Electrode and Its Application for Sensing Phenolics in Red Wines
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Synthesis of the poly(GMA)-g-MWNT
2.3. Fabrication of the Enzyme Electrode Based on Carboxylic Acid-modified MWNT
2.4. Instrumentation
3. Results and Discussion
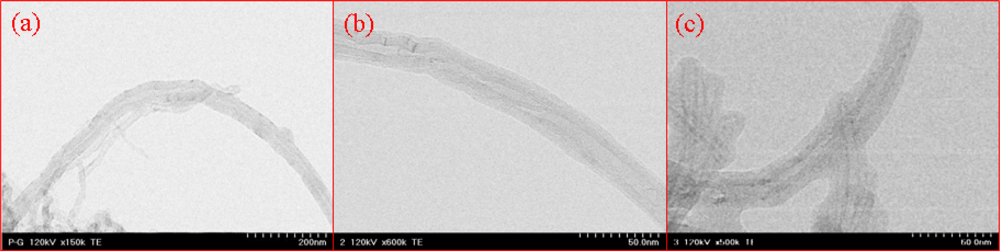
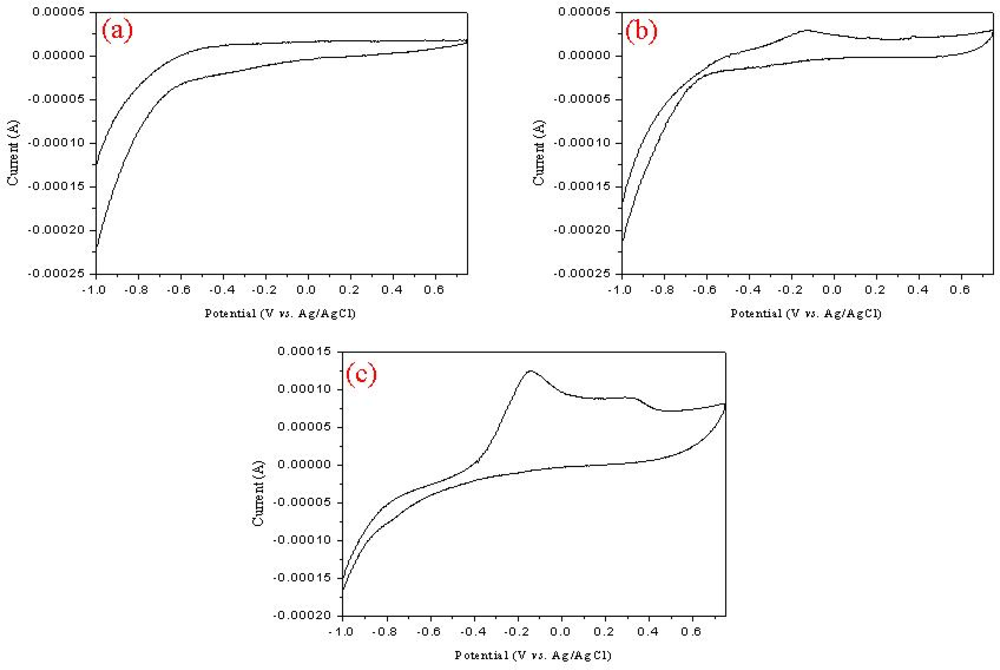
3.1. Fabrication and Characterization of MWNT Ion Liquid Electrode
3.2. Optimization of the Prepared MWNT Ion Liquid Electrode
3.3. Total Amounts of Phenolics in Commercial Red Wines
4. Conclusions
Acknowledgments
References and Notes
- Wang, J.; Musameh, M. Carbon Nanotube/Teflon Composite Electrochemical Sensors and Biosensors. Anal. Chem 2003, 75, 2075–2079. [Google Scholar]
- Wang, J.; Musameh, M.; Lin, Y.H. Solubilization of Carbon Nanotubes by Nafion toward the Preparation of Amperometric Biosensors. J. Am. Chem. Soc 2003, 125, 2408–2409. [Google Scholar]
- Saito, Y.; Yoshihawa, T. Carbon Nanocapsules Encaging Metals and Carbides. J. Phys. Chem. Solids 1993, 54, 1849–1860. [Google Scholar]
- Compton, D.L.; Laszlo, J.A. Direct Electrochemical Reduction of Hemin in Imidazolium-basedionic Liquids. J. Electroanal. Chem 2002, 520, 71–78. [Google Scholar]
- Sweeny, B.K.; Peters, D.G. Cyclic Voltammetric Study of the Catalytic Behavior of Nickel(I) Salen Electrogenerated at a Glassy Carbon Electrode in an Ionic Liquid (1-butyl-3-methylimidazolium tetrafluoroborate, BMIM+BF4−). Electrochem. Commun 2001, 3, 712–715. [Google Scholar]
- Zhao, F.; Wu, X.; Wang, M.; Lin, Y.; Gao, L.; Dong, S. Electrochemical and Bioelectrochemistry Properties of Room-Temperature Ionic Liquids and Carbon Composite Materials. Anal. Chem 2004, 76, 4960–4967. [Google Scholar]
- Li, J.; Yu, J.; Zhao, F.; Zeng, B. Direct Electrochemistry of Glucose Oxidase Entrapped in Nano Gold Particles-ionic Liquid-N,N-dimethylformamide Composite Film on Glassy Carbon Electrode and Glucose Sensing. Anal. Chem. Acta 2007, 587, 33–40. [Google Scholar]
- Sun, W.; Wang, D.; Gao, R.; Jiao, K. Direct Electrochemistry and Electrocatalysis of Hemoglobin in Sodium Alginate Film on a BMIMPF6 Modified Carbon Paste Electrode. Electrochem. Commun 2007, 9, 1159–1164. [Google Scholar]
- Safavi, A.; Maleki, N.; Moradlou, O.; Sorouri, M. Direct Electrochemistry of Hemoglobin and Its Electrocatalytic Effect based on Its Direct Immobilization on Carbon Ionic Liquid Electrode. Electrochem. Commun 2008, 10, 420–423. [Google Scholar]
- Choi, S.-H.; Lee, K.P.; Lee, J.G. Adsorption Behavior of Urokinase by Polypropylene Film Modified with Amino Acid as Affinity Group. Microchem. J 2001, 68, 205–213. [Google Scholar]
- Choi, S.-H.; Jeong, Y.H.; Ryoo, J.J.; Lee, K.P. Desalination by Electrodialysis with Ion-Exchange Membrane Prepared by Radiation-induced Graft Polymerization. Radiati. Phys. Chem 2001, 60, 503–511. [Google Scholar]
- Choi, S.-H.; Kang, H.J.; Ryu, E.N.; Lee, K.P. Electrochemical Properties of Polyolefin Nonwoven Fabric Modified with Carboxylic Acid Group for Battery Separator. Radiati. Phys. Chem 2001, 60, 495–502. [Google Scholar]
- Yang, D.-S.; Jung, D.-J.; Choi, S.-H. One-step Functionalization of Multi-walled Carbon Nanotube by Radiation-induced Graft Polymerization and Their Application of Enzyme-free Biosensors. Reac. Func. Polym. (accepted).
- Saito, K.; Kaga, T.; Yamagishi, H.; Furusaki, S. Phosphorylated Hollow Fibers Synthesized by Radiation Grafting and Cross-linking. J. Membr. Sci 1989, 43, 131–141. [Google Scholar]
- Choi, S.H.; Nho, Y.C.; Kim, G.T. Adsorption of Pb2+ and Pd2+ on Polyethylene Membrane with Amino Group Modified by Radiation-Induced Graft Copolymerization. J. Appl. Polym. Sci 1999, 71, 643–650. [Google Scholar]
- Choi, S.H.; Nho, Y.C. Adsorption of Pb2+, Cu2+, and Co2+ by Polypropylene Fabric and Polyethylene Hollow Fiber Modified by Radiation-Induced Graft Copolymerization. J. Chem. Eng 1999, 16, 241–247. [Google Scholar]
- Choi, S.H.; Nho, Y.C. Adsorption of Co2+ by Styrene-g-polyethylene Membrane Bearing Sulfonic Acid Group Modified by Radiation-Induced Graft Copolymerization. Kor. J. Appl. Polym. Sci 1999, 71, 2227–2235. [Google Scholar]
- Choi, S.H.; Nho, Y.C. Modification of Hollow Fiber Membrane with Amidoxime, Iminodiacetic Acid and Diethylene triamine by Radiation-Induced Graft Copolymerization. Kor. Polym. J 1999, 7, 38–45. [Google Scholar]
- Campanella, L.; Bonanni, A.; Finotti, E.; Tomasetti, M. Biosensors for Determination of Total and Natural Antioxidant Capacity of Red and White Wines: Comparison with other Spectrophotometric and Fluorimetric Methods. Biosens. Bioelectron 2004, 19, 641–651. [Google Scholar]
- Luximon-Ramma, A.; Bahorun, T.; Crozier, A.; Zbarsky, V.; Datla, K.P.; Dexter, D.T. Characterization of the Antioxidant Functions of Flavonoids and Proanthocyanidins in Mauritian Black Teas. Food Res. Int 2005, 38, 357–367. [Google Scholar]
- Serra, B.; Jimenez, S.; Mena, M.L.; Reviejo, A.J.; Pingarron, J.M. Composite Electrochemical Biosensors: A Comparison of Three Different Electrode Matrices for the Construction of Amperometric Tyrosinase Biosensors. Biosens. Bioelectron 2002, 17, 217. [Google Scholar]
- Tsai, Y.-C.; Chiu, C.-C. Amperometric Biosensors based on Multiwalled Carbon Nanotube-Nafion-tyrosinase Nanobiocomposites for the Determination of Phenolic Compounds. Sens. Actuats. B: Chem 2007, 125, 10–16. [Google Scholar]
- Espin, J.C.; Varon, R.; Fenoll, L.G.; Gilabert, M.A.; Garcia-Ruiz, P.A.; Tudele, J.; Canovas, F. Kinetic Characterization of the Substrate Specificity and Mechanism of Mushroom Tyrosinase. Eur. J. Biochem 2000, 22, 1270–1279. [Google Scholar]










| Sample | N (%) | C (%) | H (%) | O (%) |
|---|---|---|---|---|
| Pure MWNT | 76.14 | 0.74 | 11.81 | |
| poly(GMA)-g-MWNT | 66.78 | 4.80 | 22.25 | |
| MWNT ionic liquid | 5.35 | 55.79 | 4.63 | 18.58 |
| Commercial red wine (Korea) | Current density | Phenolics a) | Phenolics b) |
|---|---|---|---|
| Chateau Mani-dry (Korea) | 9.7×10−5 A | 383.5 mg/L | 20.33 mg/L |
| Chateau Mani-sweet (Korea) | 1.2×10−4 A | 872.9 mg/L | 38.02 mg/L |
| Chateau Mani-nouveau (Korea) | 2.1×10−4 A | 3087 mg/L | 39.01 mg/L |
© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, K.-I.; Kang, H.-Y.; Lee, J.-C.; Choi, S.-H. Fabrication of a Multi-Walled Nanotube (MWNT) Ionic Liquid Electrode and Its Application for Sensing Phenolics in Red Wines. Sensors 2009, 9, 6701-6714. https://doi.org/10.3390/s90906701
Kim K-I, Kang H-Y, Lee J-C, Choi S-H. Fabrication of a Multi-Walled Nanotube (MWNT) Ionic Liquid Electrode and Its Application for Sensing Phenolics in Red Wines. Sensors. 2009; 9(9):6701-6714. https://doi.org/10.3390/s90906701
Chicago/Turabian StyleKim, Kyo-Il, Hee-Young Kang, Jae-Chan Lee, and Seong-Ho Choi. 2009. "Fabrication of a Multi-Walled Nanotube (MWNT) Ionic Liquid Electrode and Its Application for Sensing Phenolics in Red Wines" Sensors 9, no. 9: 6701-6714. https://doi.org/10.3390/s90906701





