The Effects of Two Thick Film Deposition Methods on Tin Dioxide Gas Sensor Performance
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of SnO2 Nanoparticles
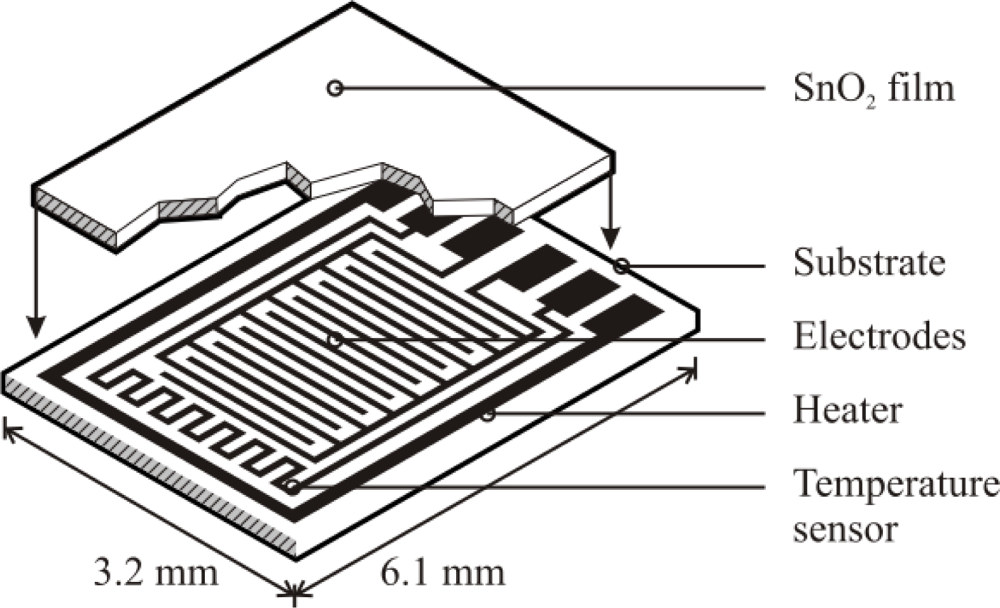
2.2. Sensor Fabrication
2.3. Sensor Testing
2.4. Materials Analysis
3. Results and Discussion
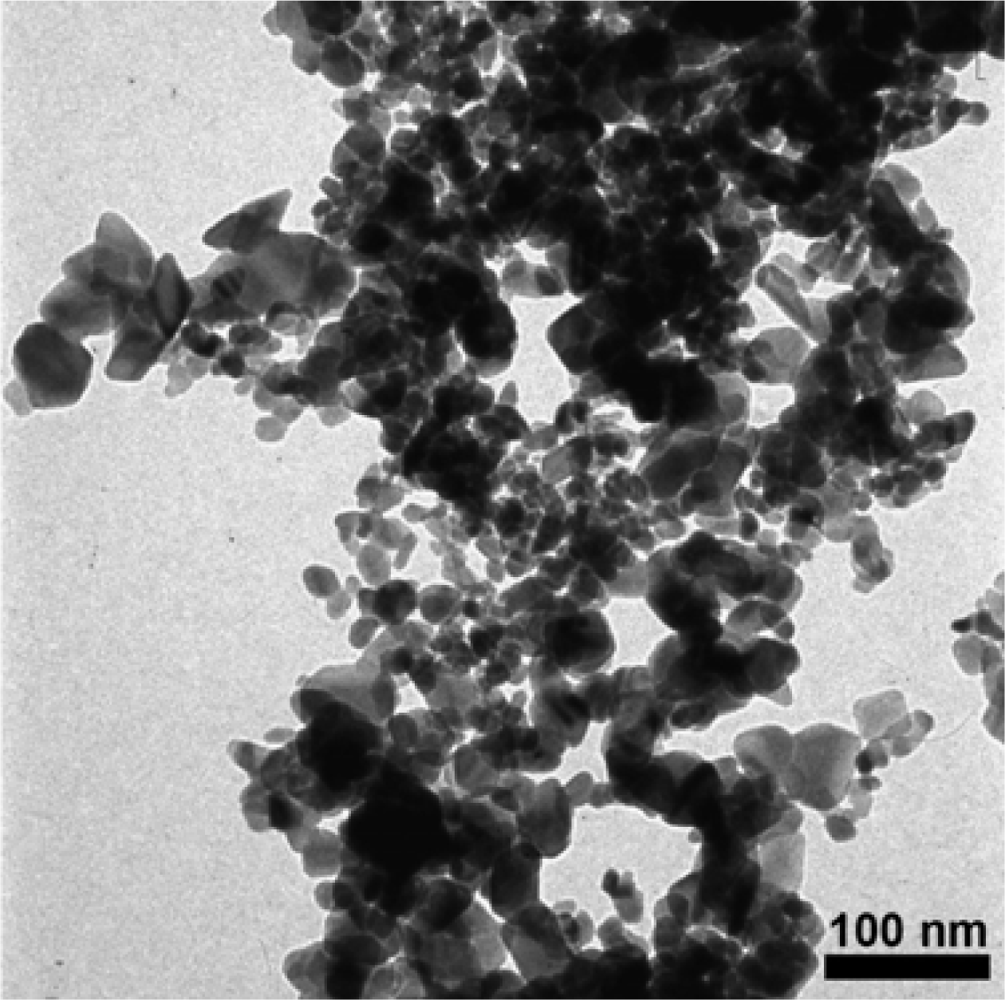
3.1. Materials Characterization of SnO2
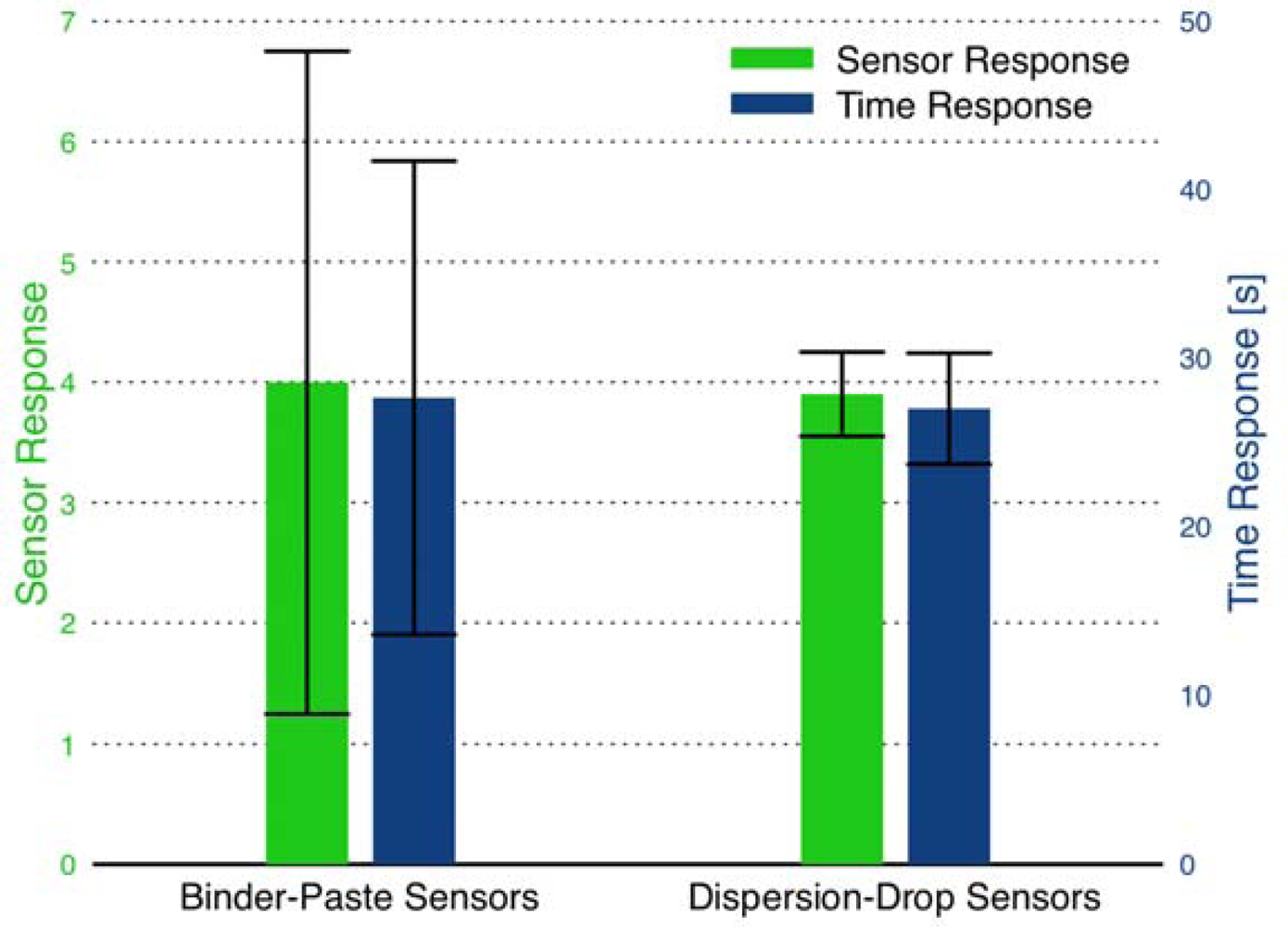
3.2. Binder-Paste Sensors
3.3. Dispersion-Drop Sensors
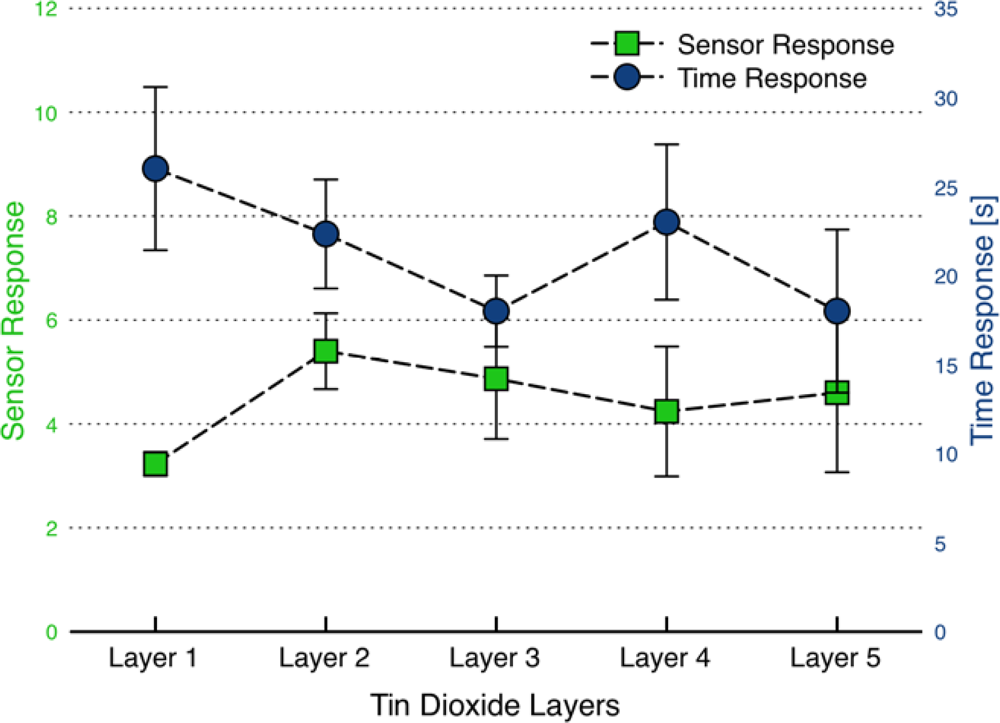
3.4. Further Characterization of the Dispersion-Drop Sensors
3.5. Comparison of Combustion-Generated and Commercial SnO2 Powders
4. Conclusions
Acknowledgments
References
- Miller, T.A.; Bakrania, S.D.; Perez, C.; Wooldridge, M.S. Nanostructured tin dioxide materials for gas sensor applications. In Functional Nanomaterials; Geckeler, K.E., Rosenberg, E., Eds.; American Scientific Publishers: Valencia, CA, USA, 2006; pp. 453–476. [Google Scholar]
- Varghese, O.K.; Grimes, C.A. Metal oxide nanoarchitectures for environmental sensing. J. Nanosci. Nanotechnol 2003, 3, 277–293. [Google Scholar]
- Wang, C.C.; Akbar, S.A.; Madou, M.J. Ceramic based resistive sensors. J. Electroceram 1998, 2, 273–282. [Google Scholar]
- Gopal Reddy, C.V.; Manorama, S.V. Room temperature hydrogen sensor based on SnO2:La2O3. J. Electrochem. Soc 2000, 147, 390–393. [Google Scholar]
- Barsan, N.; Schweizer-Berberich, M.; Göpel, W. Fresenius, fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: a status report. J. Anal. Chem 1999, 365, 287–304. [Google Scholar]
- Diéguez, A.; Romano-Rodríguez, A.; Morante, J.R.; Kappler, J.; Bârsan, N.; Göpel, W. Nanoparticle engineering for gas sensor optimisation: improved sol–gel fabricated nanocrystalline SnO2 thick film gas sensor for NO2 detection by calcination, catalytic metal introduction and grinding treatments. Sens. Actuat. B Chem 1999, 60, 125–137. [Google Scholar]
- Lee, S.; Lee, G.; Kim, J.; Kang, S.L. A novel process for fabrication of SnO2-based thick film gas sensors. Sens. Actuat. B Chem 2007, 123, 331–335. [Google Scholar]
- Durrani, S.M. Biasing voltage dependence of sensitivity of electron beam evaporated SnO2 thin film CO sensor. Sensors 2006, 6, 115–1160. [Google Scholar]
- Ando, M.; Tsuchida, T.; Miura, N.; Yamazoe, N. Influences of microstructure on hydrogen sulfide sensing characteristics of tin dioxide films. Nippon Kagaku Kaishi 1996, 4, 348–353. [Google Scholar]
- Hübner, H.P.; Obermeier, E. Reactively sputtered tin oxide thin-film gas sensors: correlation between fabrication parameters and co-sensitivity. Sens. Actuat 1989, 17, 351–354. [Google Scholar]
- Garje, D.; Aiyer, R.C. Electrical and gas-sensing properties of a thick film resistor of nanosized SnO2 with variable percentage of permanent binder. Int. J. Appl. Ceram. Technol 2006, 3, 477–484. [Google Scholar]
- Viricelle, J.P.; Riviere, B.; Pijolat, C. Optimization of SnO2 screen-printing inks for gas sensor applications. J. Eur. Ceram. Soc 2005, 25, 2137–2140. [Google Scholar]
- Ahmad, A.; Walsh, J. The influence of precursor powders and processing parameters on the properties of SnO2-based gas sensors. J. Mater. Sci 2003, 38, 4325–4332. [Google Scholar]
- Rue, G.H.; Lee, D.S.; Lee, D.D. Effects of substrates on properties of tin oxide gas sensors. Jpn. J. Appl. Phys 2004, 43, 3493–3497. [Google Scholar]
- Shimizu, Y.; Hyodo, T.; Egashira, M. Mesoporous semiconducting oxides for gas sensor application. J. Eur. Ceram. Soc 2004, 24, 1389–1398. [Google Scholar]
- Vaishnav, V.S.; Patel, P.D.; Patel, N.G. Preparation and characterization of indium tin oxide thin films for their application as gas sensors. Thin Solid Films 2005, 487, 277–282. [Google Scholar]
- Bakrania, S.D.; Miller, T.A.; Perez, C.; Wooldridge, M.S. Combustion of multiphase reactants for the synthesis of nanocomposite materials. Combust. Flame 2007, 148, 76–87. [Google Scholar]
- Bakrania, S.D.; Perez, C.; Wooldridge, M.S. Methane-assisted combustion synthesis of nanocomposite tin dioxide materials. Proc. Combust. Inst 2007, 31, 1797–1804. [Google Scholar]
- Miller, T.A.; Bakrania, S.D.; Perez, C.; Wooldridge, M.S. A new method for direct preparation of tin dioxide nanocomposite materials. J. Mater. Res 2005, 20, 2977–2987. [Google Scholar]
- Yasunaga, S.; Sunahara, S.; Ihokura, K. Effects of tetraethyl orthosilicate binder on the characteristics of an SnO2 ceramic-type semiconductor gas sensor. Sens. Actuat 1986, 9, 133–145. [Google Scholar]
- Ihokura, K.; Watson, J. The Stannic Oxide Gas Sensor - Principles and Applications; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Guidi, V.; Butturi, M.A.; Carotta, M.C.; Cavicchi, B.; Ferronia, M.; Malagu, C.; Martinellia, G.; Vincenzia, D.; Sacerdoti, M.; Zen, M. Gas sensing through thick film technology. Sens. Actuat. B Chem 2002, 84, 72–77. [Google Scholar]
- JCPDS. Powder Diffraction File; International Center for Diffraction Data: Swarthmore, PA, USA, 1990. [Google Scholar]
- Watson, J.; Ihokura, K.; Coles, G.S.V. The tin dioxide gas sensor. Meas. Sci. Technol 1994, 4, 711–719. [Google Scholar]
- Saha, M.; Banerjee, A.; Halder, A.K.; Mondal, J.; Sen, A.; Maiti, H.S. Effect of alumina addition on methane sensitivity of tin dioxide thick films. Sens. Actuat. B Chem 2001, 79, 192–195. [Google Scholar]
- Becker, T.; Ahlers, S.; Bosch-v.Braunmühl, C.; Müller, G.; Kiesewetter, O. Gas sensing properties of thin- and thick-film tin-oxide materials. Sens. Actuat. B Chem 2001, 77, 55–61. [Google Scholar]
- Karthigeyan, A.; Gupta, R.P.; Burgmair, M.; Sharma, S.K.; Eisele, I. Influence of oxidation temperature, film thickness and substrate on NO2 sensing of SnO2 ultra thin films. Sens. Actuat. B Chem 2002, 87, 321–330. [Google Scholar]
- Sakai, G.; Baik, N.S.; Miura, N.; Yamazoe, N. Gas sensing properties of tin oxide thin films fabricated from hydrothermally treated nanoparticles: Dependence of CO and H2 response on film thickness. Sens. Actuat. B Chem 2001, 77, 116–121. [Google Scholar]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuat. B Chem 1991, 5, 7–19. [Google Scholar]










| Sensor A | Sensor B Recipe | Sensor C |
|---|---|---|
| 0.10 g SnO2 | 0.32 g SnO2 | 3.25 mL TEOS |
| 0.05 g hydroxypropyl cellulose | 0.32 g ethyl cellulose | 1.35 mL ethanol |
| 6.25 mL isopropyl alcohol | 2.87 g α-terpineol soln. | 0.35 mL H2O |
| 0.05 mL 4% HCl | ||
| SnO2 with 6 wt% silica | ||
| Procedure | ||
| Sonicated for 20 mins | Mixed and stirred | Made into paste |
| 4 drop-coats with syringe | drop-coated with syringe | applied using spatula |
| sintering: 2 hrs @ 600 °C | sintering: 2 hrs @ 600 °C | sintering: 2 hrs @ 500 °C |
© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bakrania, S.D.; Wooldridge, M.S. The Effects of Two Thick Film Deposition Methods on Tin Dioxide Gas Sensor Performance. Sensors 2009, 9, 6853-6868. https://doi.org/10.3390/s90906853
Bakrania SD, Wooldridge MS. The Effects of Two Thick Film Deposition Methods on Tin Dioxide Gas Sensor Performance. Sensors. 2009; 9(9):6853-6868. https://doi.org/10.3390/s90906853
Chicago/Turabian StyleBakrania, Smitesh D., and Margaret S. Wooldridge. 2009. "The Effects of Two Thick Film Deposition Methods on Tin Dioxide Gas Sensor Performance" Sensors 9, no. 9: 6853-6868. https://doi.org/10.3390/s90906853




