Exploring the CK2 Paradox: Restless, Dangerous, Dispensable
Abstract
:1. Background
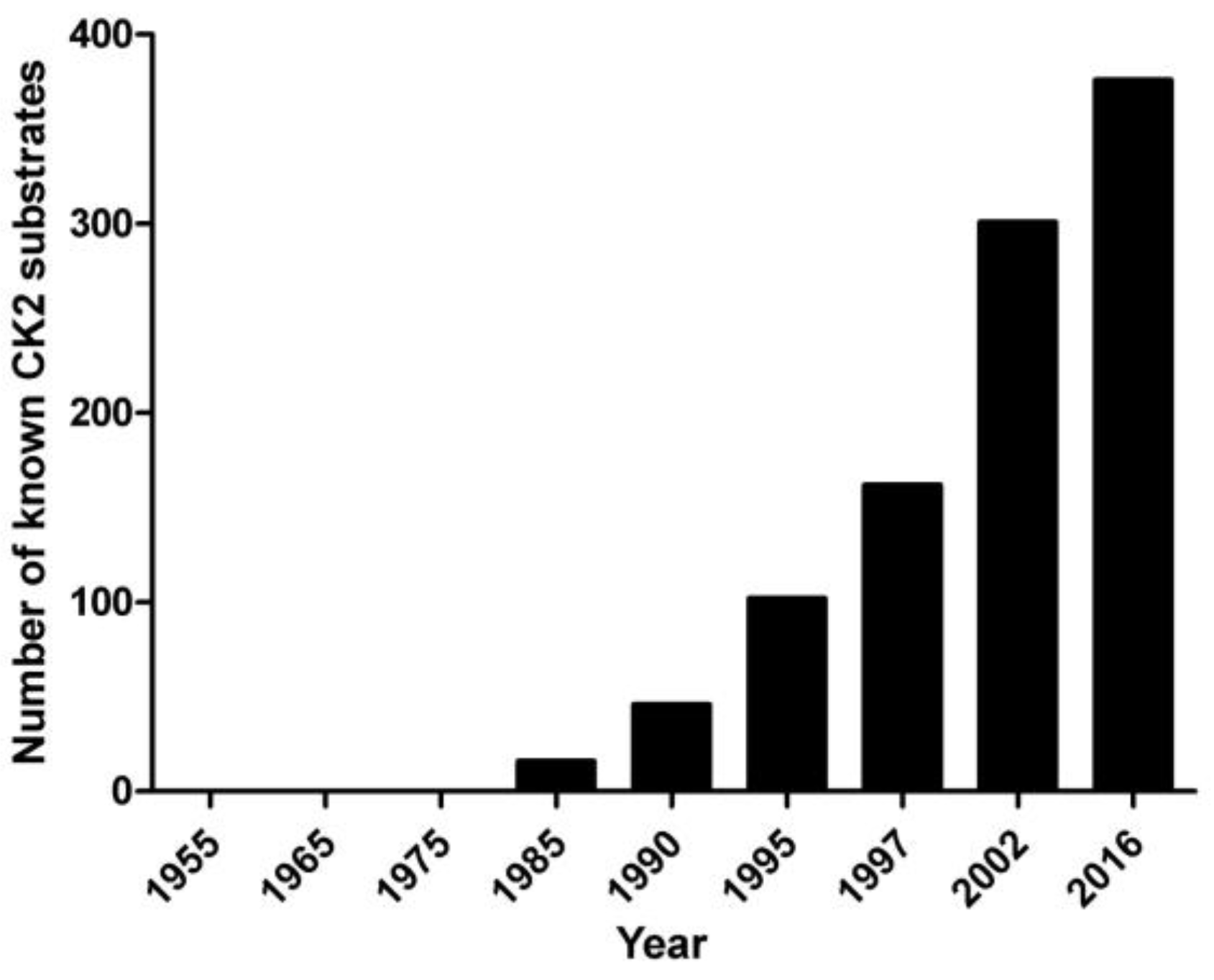
2. Pleiotropy
3. Pathogenic Potential
4. Druggability
5. Dispensability
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hammarsten, O. Zur Frage ob Caseín ein einheitlicher Stoff sei. Hoppe-Seyler Z. Physiol. Chem. 1883, 7, 227–273. [Google Scholar]
- Tagliabracci, V.S.; Engel, J.L.; Wen, J.; Wiley, S.E.; Worby, C.A.; Kinch, L.N.; Xiao, J.; Grishin, N.V.; Dixon, J.E. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science 2012, 336, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Tagliabracci, V.S.; Pinna, L.A.; Dixon, J.E. Secreted protein kinase. Trends Biochem. Sci. 2013, 38, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Tagliabracci, V.S.; Wiley, S.E.; Guo, X.; Kinch, L.N.; Durrant, E.; Wen, J.; Xiao, J.; Cui, J.; Nguyen, K.B.; Engel, J.L.; et al. A single kinase generates the majority of the secreted phosphoproteome. Cell 2015, 161, 1619–1632. [Google Scholar] [CrossRef] [PubMed]
- Burnett, G.; Kennedy, E.P. The enzymatic phosphorylation of proteins. JBC 1954, 211, 969–980. [Google Scholar]
- Dobrowolska, G.; Lozeman, F.J.; Li, D.; Krebs, E.G. CK2, a protein kinase of the next millennium. Mol. Cell. Biochem. 1999, 191, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pinna, L.A. (Ed.) Protein Kinase CK2; The Wiley-IUBMB Series on Biochemistry and Molecular Biology; John Wiley & Sons: New York, NJ, USA, 2013.
- Salvi, M.; Sarno, S.; Cesaro, L.; Nakamura, H.; Pinna, L.A. Extraordinary pleiotropy of protein kinase CK2 revealed by weblogo phosphoproteome analysis. Biochim. Biophys. Acta 2009, 1793, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Ruzzene, M.; Tosoni, K.; Zanin, S.; Cesaro, L.; Pinna, L.A. Protein kinase CK2 accumulation in “oncophilic” cells: Causes and effects. Mol. Cell. Biochem. 2011, 356, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Pinna, L.A. Casein kinase 2: An “eminence grise” in cellular regulation? Biochim. Biophys. Acta 1990, 1054, 267–284. [Google Scholar] [CrossRef]
- Pinna, L.A.; Meggio, F. Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog. Cell. Cycle Res. 1997, 3, 77–97. [Google Scholar] [PubMed]
- Meggio, F.; Pinna, L.A. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003, 17, 349–368. [Google Scholar] [CrossRef] [PubMed]
- PhosphoSitePlus. Available online: http://www.phosphosite.org (accessed on 17 November 2016).
- Montenarh, M.; Goetz, C. The interactome of Protein kinase CK2. In Protein Kinase CK2; Pinna, L.A., Ed.; John Wiley & Sons: New York, NJ, USA, 2013. [Google Scholar]
- Guerra, B.; Issinger, O.G. Protein kinase CK2 in human diseases. Curr. Med. Chem. 2008, 15, 1870–1886. [Google Scholar] [CrossRef] [PubMed]
- Ruzzene, M.; Pinna, L.A. Addiction to protein kinase CK2: A common denominator of diverse cancer cells? Biochim. Biophys. Acta 2010, 1804, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Venerando, A.; Ruzzene, M.; Pinna, L.A. Casein kinase: The triple meaning of a misnomer. Biochem. J. 2014, 460, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Issinger, O.G. Casein kinase: Pleiotropic mediators of cellular regulation. Pharmacol. Ther. 1993, 59, 1–30. [Google Scholar] [CrossRef]
- Tawfic, S.; Yu, S.; Wang, H.; Faust, R.; Davis, A.; Ahmed, K. Protein kinase CK2 signal in neoplasia. Histol. Histopathol. 2001, 16, 573–582. [Google Scholar] [PubMed]
- Trembley, J.H.; Chen, Z.; Unger, G.; Slaton, J.; Kren, B.T.; Van Waes, C.; Ahmed, K. Emergence of protein kinase CK2 as a key target in cancer therapy. Biofactors 2010, 36, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Seldin, D.C.; Leder, P. Casein kinase II alpha transgene-induced murine lymphoma: Relation to theileriosis in cattle. Science 1995, 10, 894–897. [Google Scholar] [CrossRef]
- Kelliher, M.A.; Seldin, D.C.; Leder, P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIalpha. EMBO J. 1996, 15, 5160–5166. [Google Scholar] [PubMed]
- Landesman-Bollag, E.; Channavajhala, P.L.; Cardiff, R.D.; Seldin, D.C. p53 deficiency and misexpression of protein kinase CK2α collaborate in the development of thymic lymphomas in mice. Oncogene 1998, 16, 2965–2974. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.E.; Seidner, Y.; Dominguez, I. Mining CK2 in cancer. PLoS ONE 2014, 9, e115609. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.A.; Wang, G.; Unger, G.; Slaton, J.; Ahmed, K. Protein kinase CK2—A key suppressor of apoptosis. Adv. Enzyme Regul. 2008, 48, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Widmaier, D.; Montenarh, M.; Menger, M.D.; Laschke, M.W. Protein kinase CK2 regulates Leukocyte-endothelial cell interactions during ischemia and reperfusion in striated skin muscle. Eur. Surg. Res. 2016, 57, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Ka, S.O.; Hwang, H.P.; Jang, J.H.; Hyuk Bang, I.; Bae, U.J.; Yu, H.C.; Cho, B.H.; Park, B.H. The protein kinase 2 inhibitor tetrabromobenzotriazole protects against renal ischemia reperfusion injury. Sci. Rep. 2015, 5, 14816. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Müller, I.; Dahmke, I.N.; Eichler, H.; Montenarh, M.; Menger, M.D.; Laschke, M.W. Role of protein kinase CK2 in the dynamic interaction of platelets, leukocytes and endothelial cells during thrombus formation. Thromb. Res. 2015, 136, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Komada, Y.; Hayashi, T.; Suzuki, K.; Ido, M. Protease activated receptor 1 activation of platelet is associated with an increase in protein kinase CK2 activity. J. Thromb. Haemost. 2008, 6, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Toyoda, H.; Tanaka, S.; Yamamoto, H.; Komada, Y.; Gabazza, E.C.; Hayashi, T.; Suzuki, K.; Ido, M. Phosphoinositide 3-kinase induced activation and cytoskeletal translocation of protein kinase CK2 in protease activated receptor 1-stimulated platelets. Thromb. Res. 2010, 126, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Ruiz de Azua, I.; Barella, L.F.; Sakamoto, W.; Zhu, L.; Cui, Y.; Lu, H.; Rebholz, H.; Matschinsky, F.M.; Doliba, N.M.; et al. CK2 acts as a potent negative regulator of receptor-mediated insulin release in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, E6818–E6824. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Rudzitis-Auth, J.; Dahmke, I.N.; Rössler, O.G.; Thiel, G.; Montenarh, M.; Menger, M.D.; Laschke, M.W. Inhibition of protein kinase CK2 suppresses tumor necrosis factor (TNF)-α-induced leukocyte-endothelial cell interaction. Biochim. Biophys. Acta 2015, 1852, 2123–2136. [Google Scholar] [CrossRef] [PubMed]
- Cozza, G.; Pinna, L.A. Casein kinase as potential therapeutic targets. Expert Opin. Ther. Targets 2016, 20, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Buchou, T.; Vernet, M.; Blond, O.; Jensen, H.H.; Pointu, H.; Olsen, B.B.; Cochet, C.; Issinger, O.G.; Boldyreff, B. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol. Cell. Biol. 2003, 23, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Lou, D.Y.; Dominguez, I.; Toselli, P.; Landesman-Bollag, E.; O’Brien, C.; Seldin, D.C. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol. Cell. Biol. 2008, 28, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui-Jain, A.; Drygin, D.; Streiner, N.; Chua, P.; Pierre, F.; O’Brien, S.E.; Bliesath, J.; Omori, M.; Huser, N.; Ho, C.; et al. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010, 70, 10288–10298. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.J.; Bae, K.J.; Lee, Y.; Kim, J. The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies. Front. Pharmacol. 2015, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Borgo, C.; Franchin, C.; Donella-Deana, A.; Arrigoni, G.; Pinna, L.A. Life without CK2: Generation of the first viable mammalian cell line deprived of CK2 catalytic activity. In Proceedings of the 8th International Conference on Protein Kinase CK2, Homburg, Germany, 6–9 September 2016; p. 92.
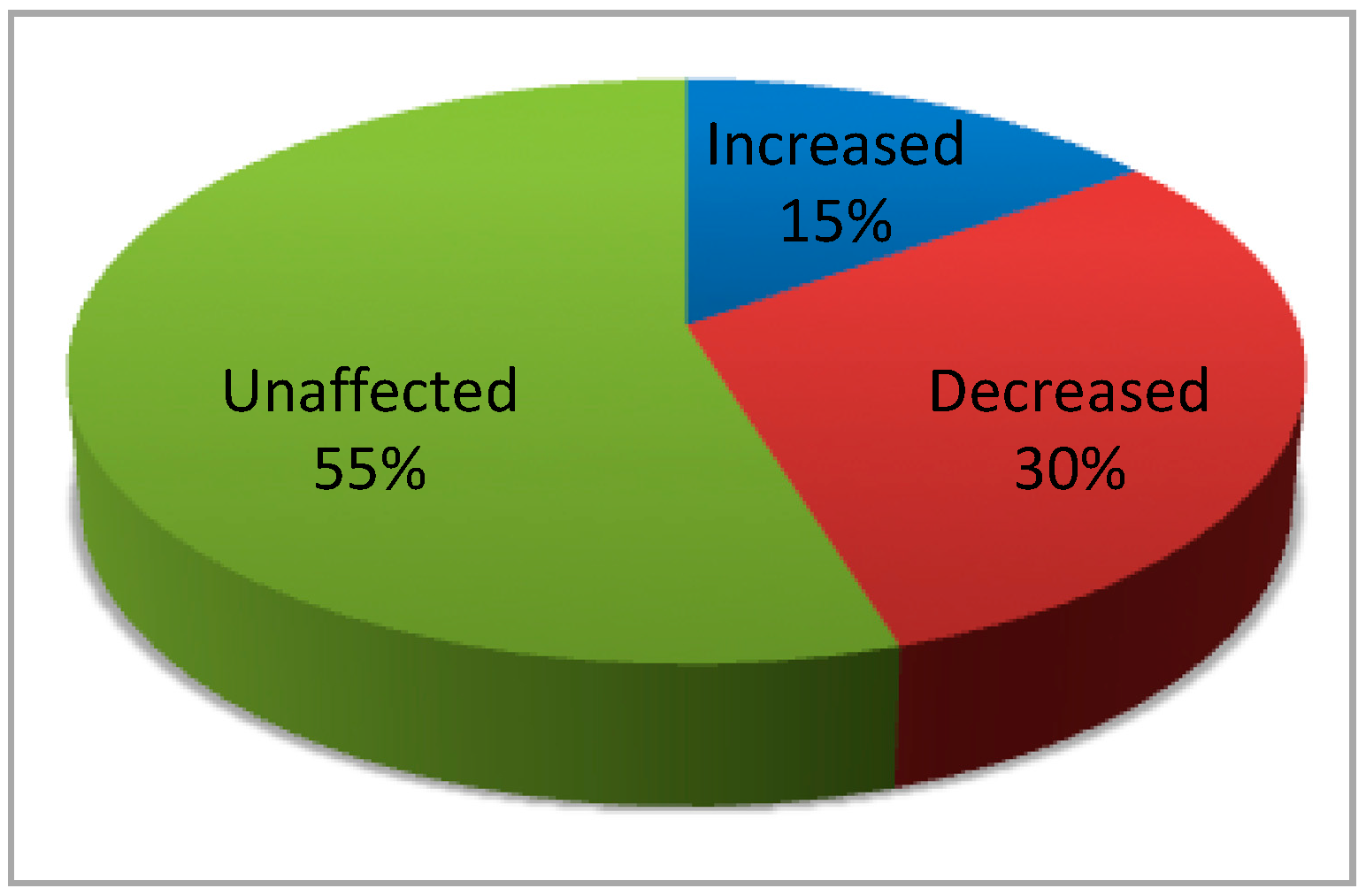
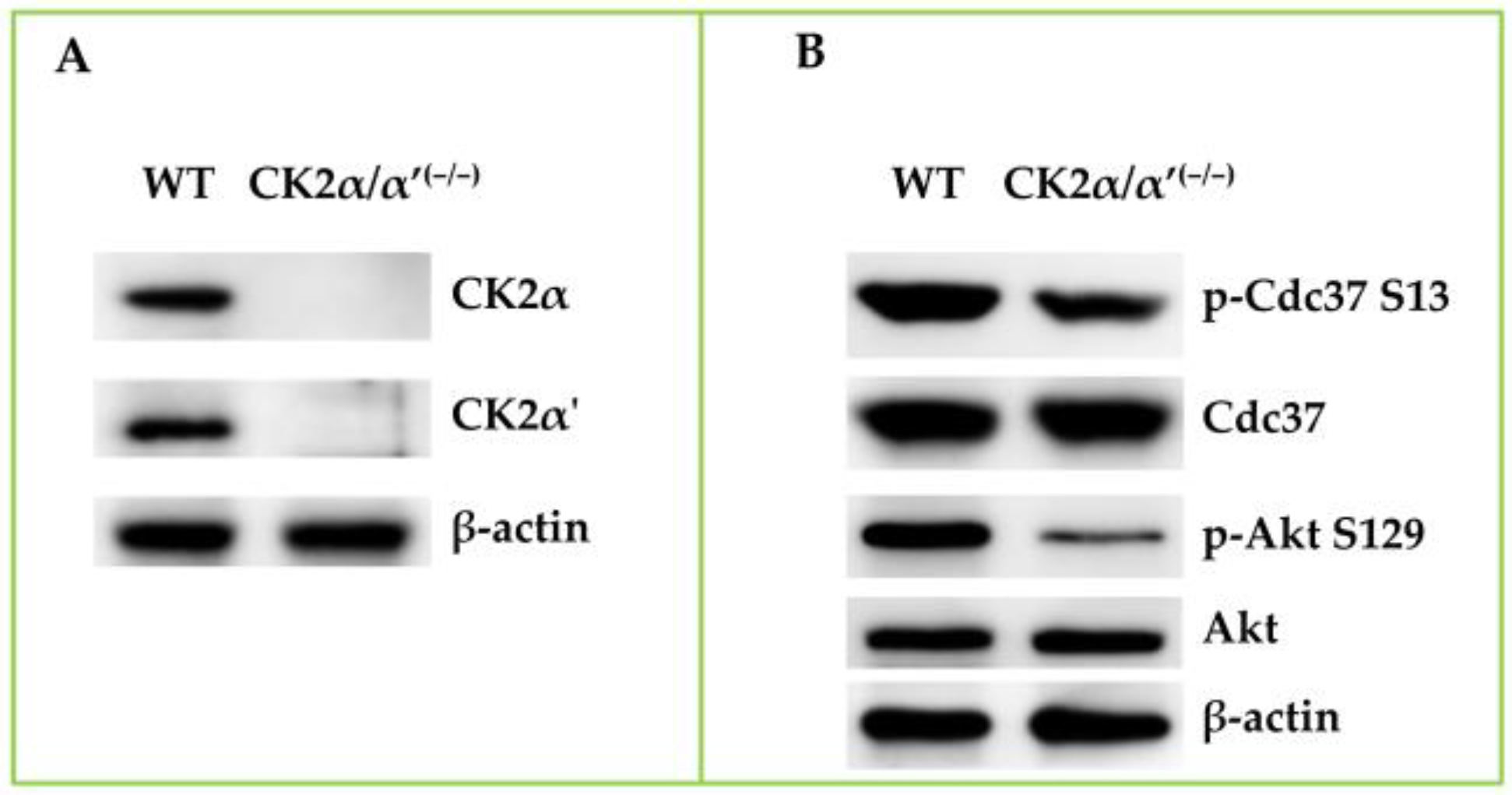
- Borgo, C.; Franchin, C.; Scalco, S.; Donella-Deana, A.; Arrigoni, G.; Salvi, M.; Pinna, L.A. Generation and quantitative proteomics analysis of CK2α/α’(−/−) cells. Sci. Rep. 2017, 7, 42409. [Google Scholar] [CrossRef]
- Kim, H.; Choi, K.; Kang, H.; Lee, S.Y.; Chi, S.W.; Lee, M.S.; Song, J.; Im, D.; Choi, Y.; Cho, S. Identification of a novel function of CX-4945 as a splicing regulator. PLoS ONE 2014, 9, e94978. [Google Scholar] [CrossRef] [PubMed]
- Franchin, C.; Cesaro, L.; Salvi, M.; Millioni, R.; Iori, E.; Cifani, P.; James, P.; Arrigoni, G.; Pinna, L.A. Quantitative analysis of a phosphoproteome readily altered by the protein kinase CK2 inhibitor quinalizarin in HEK-293T cells. Biochim. Biophys. Acta 2015, 1854, 609–623. [Google Scholar] [CrossRef] [PubMed]
| Kinase | p-Sites | p-Sites with CK2 Consensus | % |
|---|---|---|---|
| PKACA | 834 | 201 | 24.10 |
| CK2A1 | 640 | 483 | 75.46 |
| PKCA | 637 | 115 | 18.05 |
| CDK2 | 588 | 94 | 15.98 |
| CDK1 | 569 | 86 | 15.11 |
| ERK2 | 497 | 78 | 15.69 |
| ERK1 | 375 | 65 | 17.33 |
| Akt1 | 325 | 73 | 22.46 |
| GSK3B | 322 | 89 | 27.63 |
| ATM | 232 | 64 | 27.58 |
| PLK1 | 229 | 70 | 30.56 |
| P38A | 226 | 45 | 19.91 |
| CAMK2A | 216 | 50 | 23.14 |
| Chk1 | 205 | 74 | 36.09 |
| CDK5 | 190 | 20 | 10.52 |
| JNK1 | 187 | 43 | 22.99 |
| PKCD | 156 | 24 | 15.38 |
| AurB | 153 | 35 | 22.87 |
| AMPKA1 | 145 | 36 | 24.82 |
| PKCB | 145 | 27 | 18.62 |
| CK1A | 139 | 60 | 43.16 |
| DNAPK | 117 | 38 | 32.47 |
| PKCE | 104 | 20 | 19.23 |
| mTOR | 99 | 26 | 26.26 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchin, C.; Borgo, C.; Zaramella, S.; Cesaro, L.; Arrigoni, G.; Salvi, M.; Pinna, L.A. Exploring the CK2 Paradox: Restless, Dangerous, Dispensable. Pharmaceuticals 2017, 10, 11. https://doi.org/10.3390/ph10010011
Franchin C, Borgo C, Zaramella S, Cesaro L, Arrigoni G, Salvi M, Pinna LA. Exploring the CK2 Paradox: Restless, Dangerous, Dispensable. Pharmaceuticals. 2017; 10(1):11. https://doi.org/10.3390/ph10010011
Chicago/Turabian StyleFranchin, Cinzia, Christian Borgo, Silvia Zaramella, Luca Cesaro, Giorgio Arrigoni, Mauro Salvi, and Lorenzo A. Pinna. 2017. "Exploring the CK2 Paradox: Restless, Dangerous, Dispensable" Pharmaceuticals 10, no. 1: 11. https://doi.org/10.3390/ph10010011







