188Re(V) Nitrido Radiopharmaceuticals for Radionuclide Therapy
Abstract
:1. Introduction
2. Rhenium-188
The Parallelism between Technetium-99m and Rhenium-188 Radiopharmaceuticals Preparation
3. Design of 188Re Radiopharmaceuticals
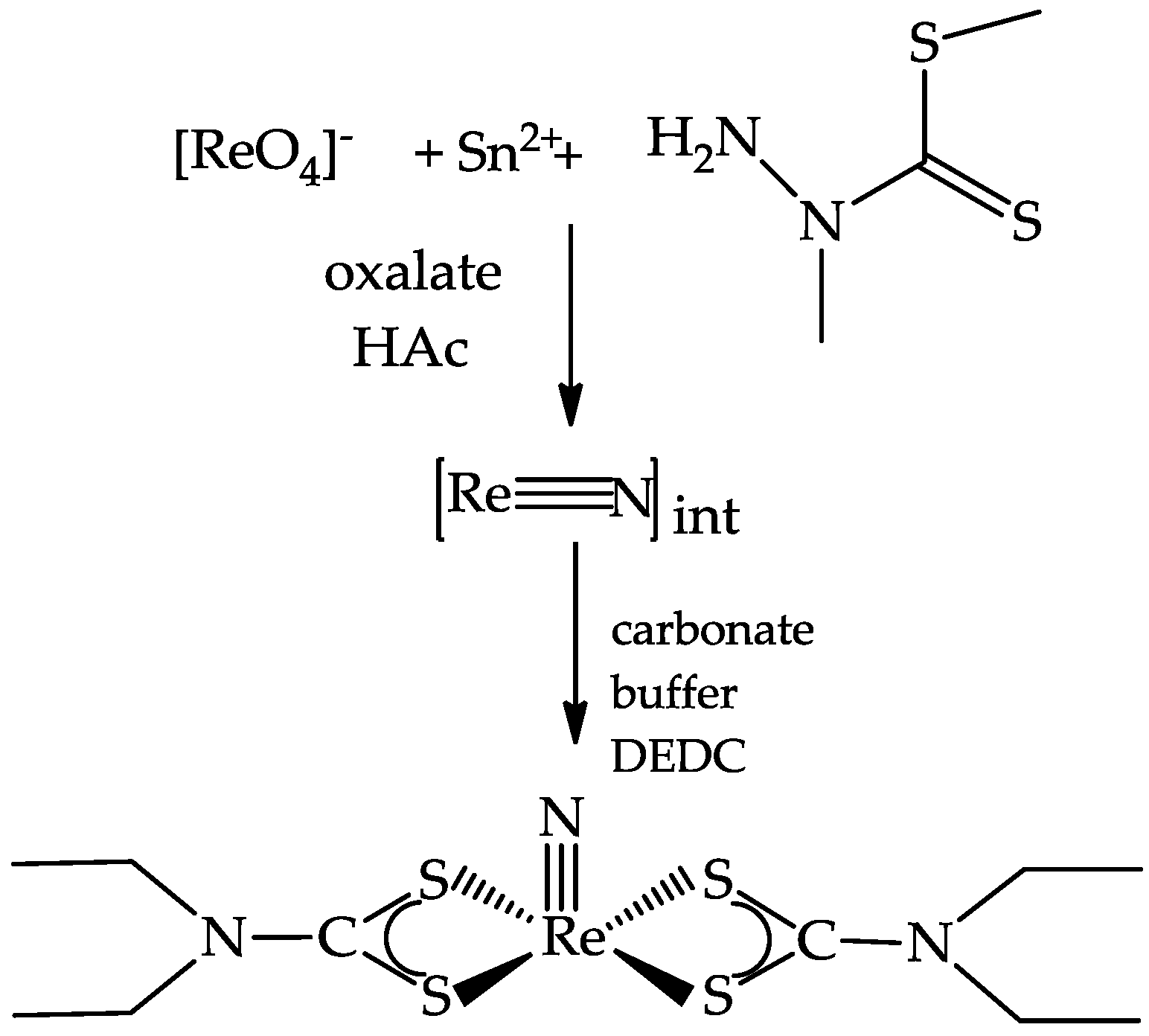
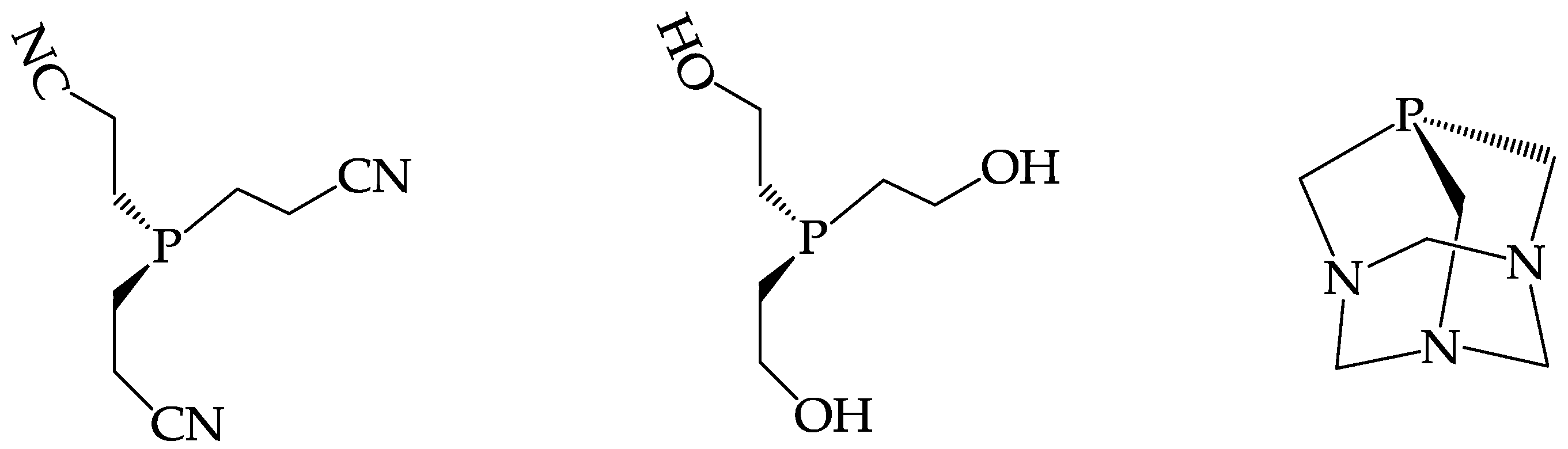
3.1. The Preparation of 188Re(V) Nitrido Radiopharmaceuticals
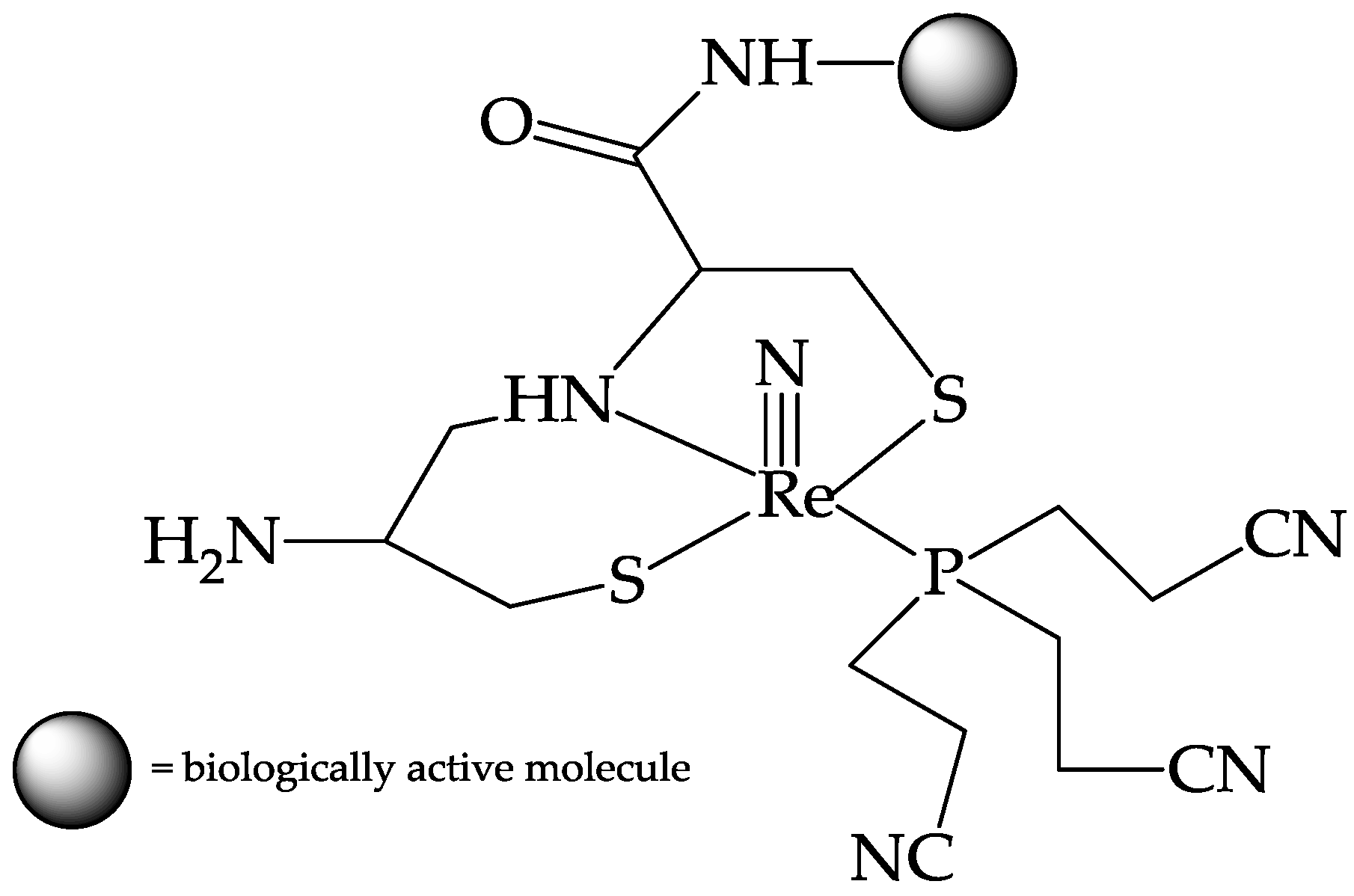
3.2. The Labelling of Bioactive Molecules with the 188ReN Core
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dash, A.; Knapp, F.F.; Pillai, M.R. Targeted radionuclide therapy-an overview. Curr. Radiopharm. 2013, 6, 152–180. [Google Scholar] [CrossRef] [PubMed]
- Preston, R.J. Radiation biology: Concepts for radiation protection. Health Phys. 2005, 88, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Sledge, C.B.; Zuckerman, J.D.; Zalutsky, M.R.; Atcher, R.W.; Shortkroff, S.; Lionberger, D.R.; Rose, H.A.; Hurson, B.J.; Lankenner, P.A.; Anderson, R.J., Jr.; et al. Treatment of rheumatoid synovitis of the knee with intraarticular injection of dysprosium 165-ferric hydroxide macroaggregates. Arthrits Rheum. 1986, 29, 153–159. [Google Scholar] [CrossRef]
- Brans, B.; Linden, O.; Giammarile, F.; Tenvall, J.; Punt, C. Clinical applications of newer radionuclide therapies. Eur. J. Cancer 2006, 42, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, M.; Ferrari, M.; Di Dia, A.; Botta, F.; De Cicco, C.; Bodei, L.; Paganelli, G. Recent issues on dosimetry and radiobiology for peptide receptor radionuclide therapy. Q. J. Nucl. Med. Mol. Imaging 2011, 55, 155–167. [Google Scholar] [PubMed]
- Zaknun, J.J.; Bodei, L.; Mueller-Bran, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Flores, G.; de Murphy, C.A. Pharmacokinetics and dosimetry of 188Re-pharmaceuticals. Adv. Drug Deliv. Rev. 2008, 60, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I.; Adelstein, J. Considerations in the selection of radionuclides for cancer therapy. In Handbook of Radiopharmaceuticals, Radiochemistry and Applications; Welch, M.J., Redvanly, C.S., Kassis, A.I., Adelstein, S.J., Eds.; Wiley & Sons: London, UK, 2003; pp. 767–793. [Google Scholar]
- Frier, M. Rhenium-188 and Copper-67 Radiopharmaceuticals for the Treatment of Bladder Cancer. Mini. Rev. Med. Chem. 2004, 4, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Sattelberger, A.P.; Bryan, J.C. Comprehensive Organometallic Chemistry II; Elsevier: Amsterdam, The Netherlands, 1994; Volume 4. [Google Scholar]
- Jackel, B.; Cripps, R.; Guntay, S.; Bruchertseifer, H. Development of semiautomated system for the preparation of 188Re aqueous solutions of high and reproducible activity concentrations. Appl. Radiat. Isot. 2005, 63, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Mansur, M.S.; Mushtaq, A.; Jehangir, M. Concentration of 99mTc-pertechnate and 188Re-perrrhenate. Radiochim. Acta 2006, 94, 107–111. [Google Scholar] [CrossRef]
- Mushtaq, A. Concentration of 99mTcO4−/188ReO4− by a single, compact, anion exchange cartridge. Nucl. Med. Commun. 2004, 25, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K.; Venkatesh, M.; Ramamoorthy, N. Evaluation of two methods of concentrating perrhanate (188Re) eluates from 188W-188Re generator. Appl. Radiat. Isot. 2009, 67, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Tanase, M.; Tatenuma, K.; Ishikawa, K.; Kurosawa, K.; Nishino, M.; Hasegawa, Y. A 99mTc generator using a new inorganic polymer adsorbent for (n,γ )99Mo. Appl. Radiat. Isot. 1997, 48, 607–611. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Pasquali, M.; Duatti, A.; Taibi, A.; Pupillo, G.; Esposito, J. 188W/188Re Generator System and Its Therapeutic Applications. J. Chem. 2014, 2014, 529406. [Google Scholar] [CrossRef]
- Dadachov, M.; Lambrecht, R.M. 188W-188Re gel generators based on metal tungstates. J. Radioanal. Nucl. Chem. 1995, 200, 211–221. [Google Scholar] [CrossRef]
- Dadachov, M.; Lambrecht, R.M.; Hetheringtion, E. An improved tungsten-188/rhenium-188 gel generator based on zirconium tungstate. J. Radioanal. Nucl. Chem. 1994, 184, 267–278. [Google Scholar] [CrossRef]
- Arano, Y. Recent advances in 99mTc radiopharmaceuticals. Ann. Nucl. Med. 2002, 16, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, L.; Boschi, A.; Pasquali, M.; Duatti, A.; Di Domenico, G.; Pupillo, G.; Esposito, J.; Giganti, M.; Taibi, A.; Gambaccini, M. Influence of the generator in-growth time on the final radiochemical purity and stability of Tc-99m radiopharmaceuticals. Sci. Technol. Nucl. Ins. 2013, 2013, 379283. [Google Scholar] [CrossRef]
- Esposito, J.; Vecchi, G.; Pupillo, G.; Taibi, A.; Uccelli, L.; Boschi, A.; Gambaccini, M. Evaluation of 99Mo and 99mTc productions based on a high-performance cyclotron. Sci. Technol. Nucl. Ins. 2013. [Google Scholar] [CrossRef]
- Deutsch, E.; Libson, K.; Vanderheyden, J.-L.; Ketring, A.R.; Maxon, H.R. The chemistry of rhenium and technetium as related to the use of isotopes of these elements in therapeutic and diagnostic nuclear medicine. Nucl. Med. Biol. 1986, 13, 465–477. [Google Scholar] [CrossRef]
- Xia, J.; Wang, Y.; Yu, J.; Li, S.; Tang, L.; Zheng, M.; Liu, X.; Li, G.; Cheng, D.; Liang, S.; Yin, D. Synthesis, in vitro and in vivo behavior of 188Re(I)-tricarbonyl complexes for the future functionalization of biomolecules. J. Radioanal. Nucl. Chem. 2008, 275, 325–330. [Google Scholar] [CrossRef]
- Fernandes, C.; Monteiro, S.; Belchior, A.; Marques, F.; Gano, L.; Correia, J.D.; Santos, I. Novel 188Re multi-functional bone-seeking compounds: Synthesis, biological and radiotoxic effects in metastatic breast cancer cells. Nucl. Med. Biol. 2016, 43, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Comazzi, V.; Bellande, E.; Duatti, A.; Marchi, A. A new efficient method for the preparation of 99mTc radiopharmaceuticals containing the TcN multiple bond. Appl. Radiat. Isot. 1992, 43, 1329–1333. [Google Scholar] [CrossRef]
- Boschi, A.; Bolzati, C.; Uccelli, L.; Duatti, A. High-yield synthesis of the terminal 188ReN multiple bond from generator produced [188ReO4]−. Nucl. Med. Biol. 2003, 30, 381–387. [Google Scholar] [CrossRef]
- Pasqualini, R.; Bellande, E.; Comazzi, V.; Duatti, A. Improved synthesis of 99mTc nitrido dithiocarbamate myocardial imaging agents. J. Nucl. Med. 1992, 33, 989–990. [Google Scholar]
- Pasqualini, R.; Duatti, A.; Bellande, E.; Comazzi, V.; Brucato, V.; Hoffschir, D.; Fagret, D.; Comet, M. A Class of Neutral Myocardial Imaging Agents. J. Nucl. Med. 1994, 35, 334–341. [Google Scholar] [PubMed]
- Cittanti, C.; Uccelli, L.; Pasquali, M.; Boschi, A.; Flammia, C.; Bagatin, E.; Casali, M.; Stabin, M.G.; Feggi, L.; Giganti, M.; et al. Whole-Body Biodistribution and Radiation Dosimetry of the New Cardiac Tracer 99mTc-N-DBODC. J. Nucl. Med. 2008, 49, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Keng, G.H.; Sundram, F.X. Radionuclide therapy of hepatocellular carcinoma. Ann. Acad. Med. Singap. 2003, 32, 518–524. [Google Scholar] [PubMed]
- Lee, Y.S.; Jeong, J.M.; Kim, Y.J.; Chung, J.W.; Park, J.H.; Suh, Y.G.; Lee, D.S.; Chung, J.K.; Lee, M.C. Synthesis of 188Re-labelled long chain alkyl diaminedithiol for therapy of liver cancer. Nucl. Med. Commun. 2002, 23, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.M.; Kim, Y.J.; Lee, Y.S.; Ko, J.I.; Son, M.; Lee, D.S.; Chung, J.K.; Park, J.H.; Lee, M.C. Lipiodol solution of a lipophilic agent, 188Re-TDD, for the treatment of liver cancer. Nucl. Med. Biol. 2001, 28, 197–204. [Google Scholar] [CrossRef]
- Sundram, F.X.; Yu, S.W.; Jeong, J.M.; Somanesan, S.; Premaraj, J.; Saw, M.M.; Tan, B.S. 188Rhenium-TDD-lipiodol in treatment of inoperable primary hepatocellular carcinoma—A case report. Ann. Acad. Med. Singap. 2001, 30, 542–545. [Google Scholar] [PubMed]
- Paeng, J.C.; Jeong, J.M.; Yoon, C.J.; Lee, Y.S.; Suh, Y.G.; Chung, J.W.; Park, J.H.; Chung, J.K.; Son, M.; Lee, M.C. Lipiodol solution of 188Re-HDD as a new therapeutic agent for transhepatic arterial embolization in liver cancer: Preclinical study in a rabbit liver cancer model. J. Nucl. Med. 2003, 44, 2033–2038. [Google Scholar]
- Keng, G.H.; Sundram, F.X.; Yu, S.W.; Somanesan, S.; Premaraj, J.; Oon, C.J.; Kwok, R.; Htoo, M.M. Preliminary experience in radionuclide therapy of hepatocellular carcinoma using hepatic intra-arterial radio-conjugates. Ann. Acad. Med. Singap. 2002, 31, 382–386. [Google Scholar] [PubMed]
- Boschi, A.; Uccelli, L.; Duatti, A.; Colamussi, P.; Cittanti, C.; Filice, A.; Rose, A.H.; Martindale, A.A.; Claringbold, P.G.; Kearney, D.; et al. A kit formulation for the preparation of 188Re-lipiodol: Preclinical studies and preliminary therapeutic evaluation in patients with unresectable hepatocellular carcinoma. Nucl. Med. Commun. 2004, 25, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, L.; Pasquali, M.; Boschi, A.; Giganti, M.; Duatti, A. Automated preparation of Re-188 lipiodol for the treatment of hepatocellular carcinoma. Nucl. Med. Biol. 2011, 38, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Boschi, A.; Ravani, L.; Cortesi, R.; Drechsler, M.; Mariani, P.; Moscatelli, S.; Contado, C.; Di Domenico, G.; Nastruzzi, C.; et al. Biodistribution of nanostructured lipid carriers: A tomographic study. Eur. J. Pharm. Biopharm. 2015, 89, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Hom, R.K.; Katzenellenbogen, J.A. Technetium-99m-labeled receptor-specific small-molecule radiopharmaceuticals (Recent developments and encouraging results). Nucl. Med. Biol. 1997, 24, 485–498. [Google Scholar] [CrossRef]
- Guozheng, L.; Hnatowich, D.J. Labeling biomolecules with radiorhenium—A review of the bifunctional chelators. Anticancer Agents Med Chem. 2007, 7, 367–377. [Google Scholar]
- Alberto, R.; Schibli, R.; Angst, D.; Schubiger, P.A.; Abram, U.; Abram, S.; Kaden, T.A. Application of technetium and rhenium carbonyl chemistry to nuclear medicine. Preparation of [NEt4]2[TcCl3(CO)3] from [NBu4][TcO4] and structure of [NEt4][Tc-2(mu-Cl)3(CO)6]; structures of the model complexes [NEt4][Re-2(mu-OEt)2(mu-OAc)(CO)6] and [ReBr({-CH2S(CH2)2Cl}2)(CO)3]. Transit. Metal Chem. 1997, 2, 97–601. [Google Scholar]
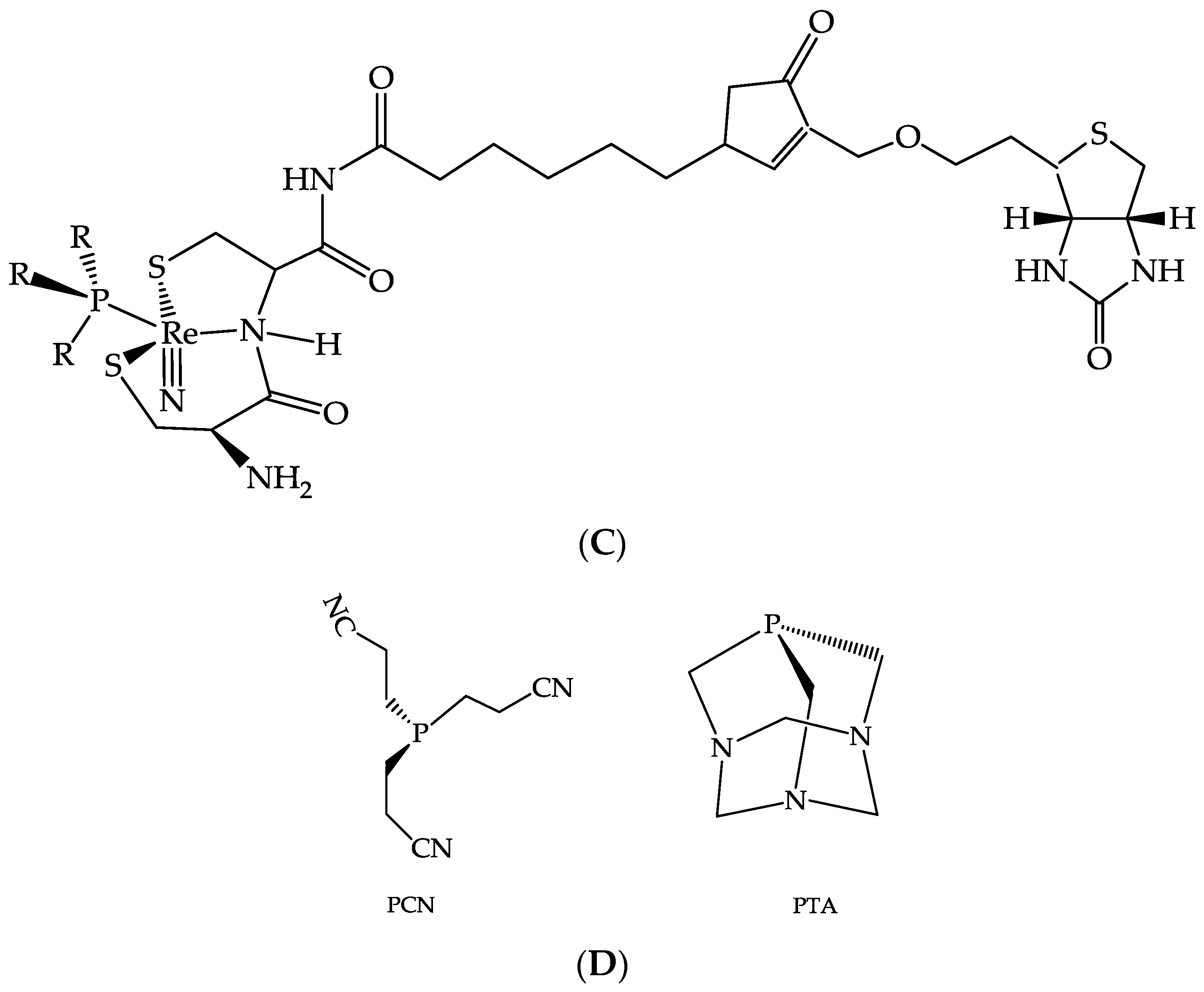
- Thieme, S.; Agostini, S.; Bergmann, R.; Pietzsch, J.; Pietzsch, H.-J.; Carta, D.; Salvarese, N.; Refosco, F.; Bolzati, C. Synthesis, characterization and biological evaluation of [188Re(N)(cys∼)(PNP)]+/0 mixed-ligand complexes as prototypes for the development of 188Re(N)-based target-specific radiopharmaceuticals. Nucl. Med. Biol. 2011, 38, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Uccelli, L.; Pasquali, M.; Pasqualini, R.; Guerrini, R.; Duatti, A. Mixed tridentate π-donor and monodentate π-acceptor ligands as chelating systems for rhenium-188 and technetium-99m nitride radiopharmaceuticals. Curr. Radiopharm. 2013, 6, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Cazzola, E.; Uccelli, L.; Pasquali, M.; Ferretti, V.; Bertolasi, V.; Duatti, A. Rhenium(V) and technetium(V) nitride complexes with mixed tridentate π-donor and monodentate π-acceptor ligands. Inorg. Chem. 2012, 51, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Giglio, J.; Rey, A.; Cerecetto, H.; Pirmettis, I.; Papadopoulos, M.; León, E.; Monge, A.; López de Ceráin, A.; Azqueta, A.; González, M.; et al. Design and evaluation of “3 + 1” mixed ligand oxorhenium and oxotechnetium complexes bearing a nitroaromatic group with potential application in nuclear medicine oncology. Eur. J. Med. Chem. 2006, 41, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Pirmettis, I.C.; Papadopoulos, M.S.; Chiotellis, E. Novel 99mTc aminobisthiolato/monothiolato “3 + 1“ mixed ligand complexes: structure-activity relationships and preliminary in vivo validation as brain blood flow imaging agents. J. Med. Chem. 1997, 40, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Pirmettis, I.C.; Papadopoulos, M.S.; Mastrostamatis, S.G.; Raptopoulou, C.P.; Terzis, A.; Chiotellis, E. Synthesis and characterization of oxotechnetium(V) mixed-ligand complexes containing a tridentate N-substituted bis(2-mercaptoethyl)amine and a monodentate thiol. Inorg. Chem. 1996, 35, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Smilkov, K.; Janevik, E.; Guerrini, R.; Pasquali, M.; Boschi, A.; Uccelli, L.; Di Domenico, G.; Duatti, A. Preparation and first biological evaluation of novel Re-188/Tc-99m peptide conjugates with substance-P. Appl. Radiat. Isot. 2014, 92, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Van Hagen, P.M.; Breeman, W.A.P.; Reubi, J.C.; Postema, P.T.E.; Van den AnkerLugtenburg, P.J.; Kwekkeboom, D.J.; Laissue, J.; Waser, B.; Lamberts, S.W.J.; Visser, T.J.; et al. Visualization of the thymus by substance P receptor scintigraphy in man. Eur. J. Nucl. Med. 2005, 23, 1508–1513. [Google Scholar] [CrossRef]
- Ansquer, C.; Kraeber-Bodéré, F.; Chatal, J. Current status and perspectives in peptide receptor radiation therapy. Curr. Pharm. Des. 2009, 15, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
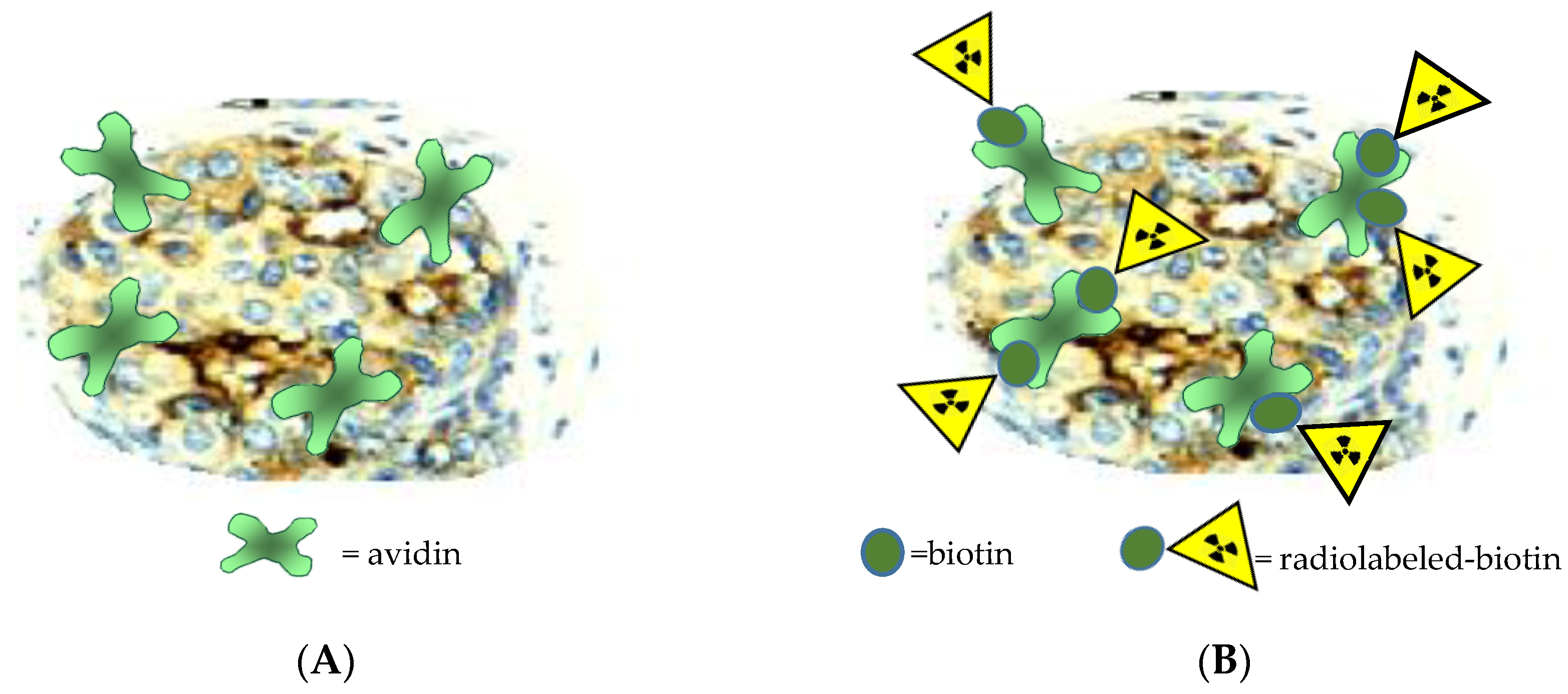
- Pasquali, M.; Janevik, E.; Uccelli, L.; Boschi, A.; Duatti, A. Labelling of biotin with 188Re. In IAEA Radioisotopes and Radiopharmaceuticals Series, Yttrium-90 and Rhenium-188 Radiopharmaceuticals for Radionuclide Therapy; IAEA: Vienna, Austria, 2015; pp. 120–133. [Google Scholar]
- James, S.; Maresca, K.P.; Allis, D.G.; Valliant, J.F.; Eckelman, W.; Babich, J.W.; Zubieta, J. Extension of the single amino acid chelate concept (SAAC) to bifunctional biotin analogues for complexation of the M(CO)3+1 core (M = Tc and Re): Syntheses, characterization, biotinidase stability, and avidin binding. Bioconjugate Chem. 2006, 17, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, B.; Makowska, M.; Domagala, A.; Rybarczyk-Pirek, A.; Zakrzewski, J. Metallo-carbonyl conjugates of biotin and biocytin. J. Organomet. Chem. 2003, 668, 95–100. [Google Scholar] [CrossRef]
- Kam-Winglo, K.; Hui, W.K.; Chun-Ming, N.G.D. Novel rhenium(I) polypyridine biotin complexes that show luminescence enhancement and lifetime elongation upon binding to avidin. J. Am. Chem. Soc. 2002, 124, 9344–9345. [Google Scholar]
- Kam-Winglo, K.; Hui, W.K. Design of rhenium(I) polypyridine biotin complexes as a new class of luminescent probes for avidin. Inorg. Chem. 2005, 44, 1992–2002. [Google Scholar]
- Duatti, A.; Boschi, A.; Pasquali, M.; Janevik, E.; Guerrini, R.; Trapella, C.; Uccelli, L. Might Re-188 be more effective for boosting therapy of breast cancer using the IART approach? Eur. J. Nucl. Med. Mol. Imaging 2010, 37, S363. [Google Scholar]
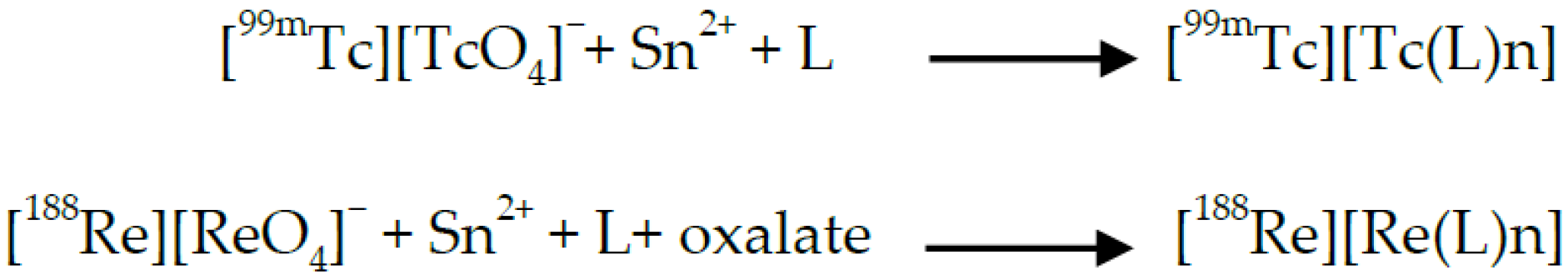

| Radionuclides | Half-Life (T1/2) | Eβmax (keV) * | Rβmax (mm) # |
|---|---|---|---|
| 67Cu | 61.9 h | 575 | 2.1 |
| 165Dy | 2.3 h | 1285 | 5.9 |
| 166Ho | 28.8 h | 1854 | 9.0 |
| 131I | 8.0 d | 606 | 2.3 |
| 90Y | 64.1 h | 2284 | 11.3 |
| 177Lu | 6.7 d | 497 | 1.8 |
| 32P | 14.3 d | 1710 | 8.2 |
| 186Re | 3.8 d | 1077 | 4.8 |
| 188Re | 17.0 h | 2120 | 10.4 |
| 89Sr | 50.5 d | 1491 | 7.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschi, A.; Martini, P.; Uccelli, L. 188Re(V) Nitrido Radiopharmaceuticals for Radionuclide Therapy. Pharmaceuticals 2017, 10, 12. https://doi.org/10.3390/ph10010012
Boschi A, Martini P, Uccelli L. 188Re(V) Nitrido Radiopharmaceuticals for Radionuclide Therapy. Pharmaceuticals. 2017; 10(1):12. https://doi.org/10.3390/ph10010012
Chicago/Turabian StyleBoschi, Alessandra, Petra Martini, and Licia Uccelli. 2017. "188Re(V) Nitrido Radiopharmaceuticals for Radionuclide Therapy" Pharmaceuticals 10, no. 1: 12. https://doi.org/10.3390/ph10010012






