Inhibition of Protein Kinase CK2 Prevents Adipogenic Differentiation of Mesenchymal Stem Cells Like C3H/10T1/2 Cells
Abstract
:1. Introduction
2. Results
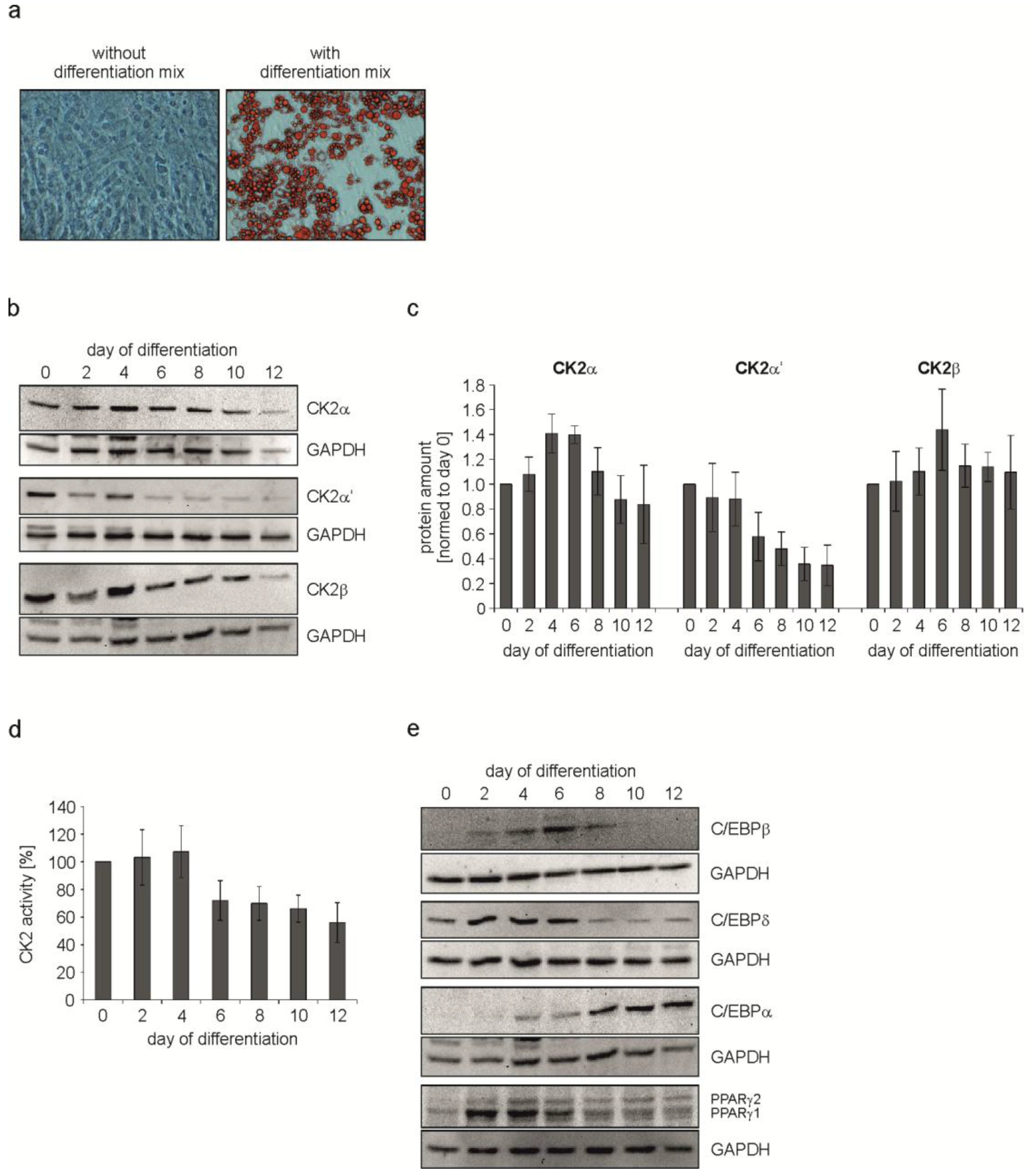
2.1. Characterization of CK2 and Adipogenic Transcription Factors during C3H/10T1/2 Differentiation
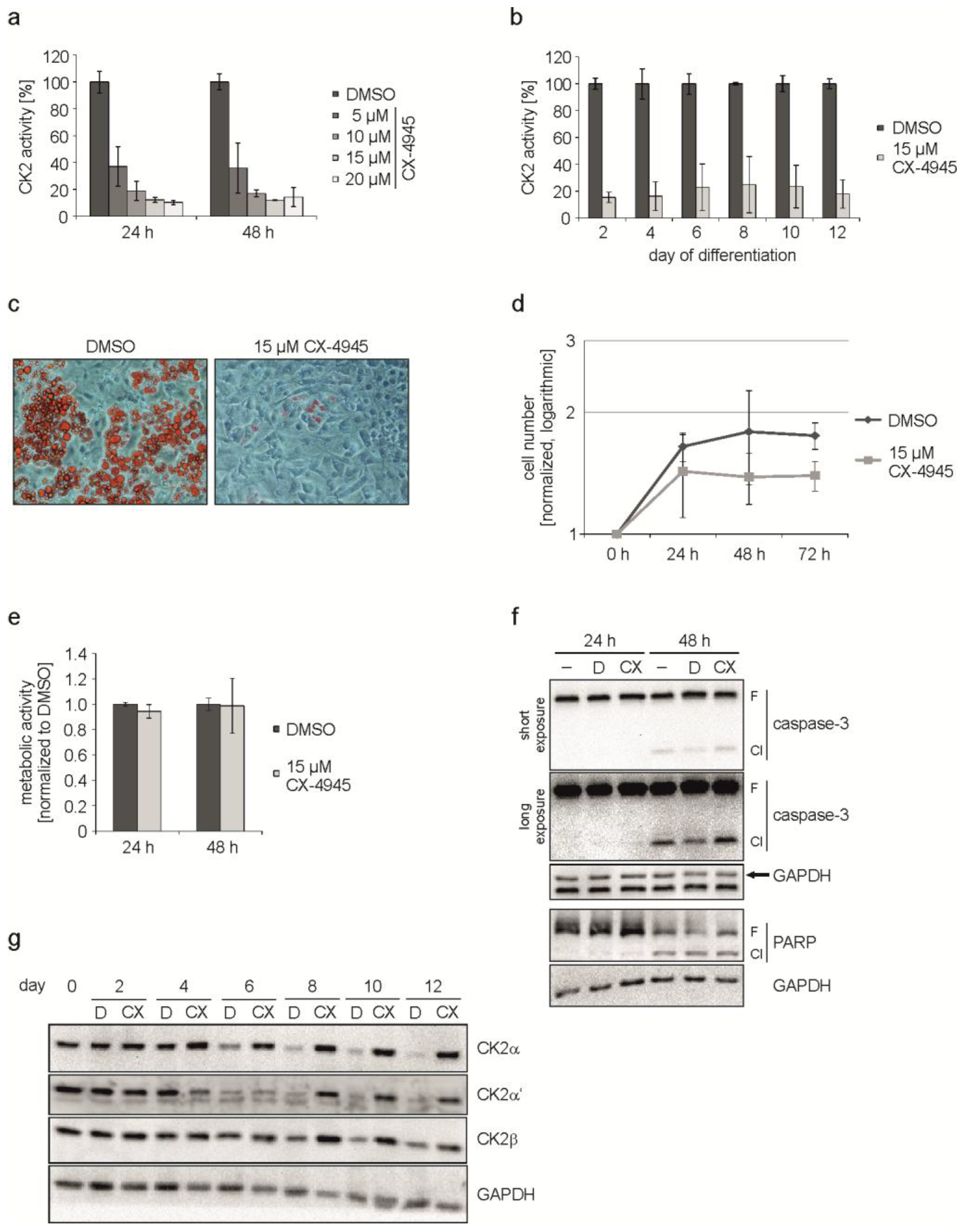
2.2. Inhibition of CK2 Kinase Activity with CX-4945 Influences Differentiation, Proliferation, and CK2 Protein Expression
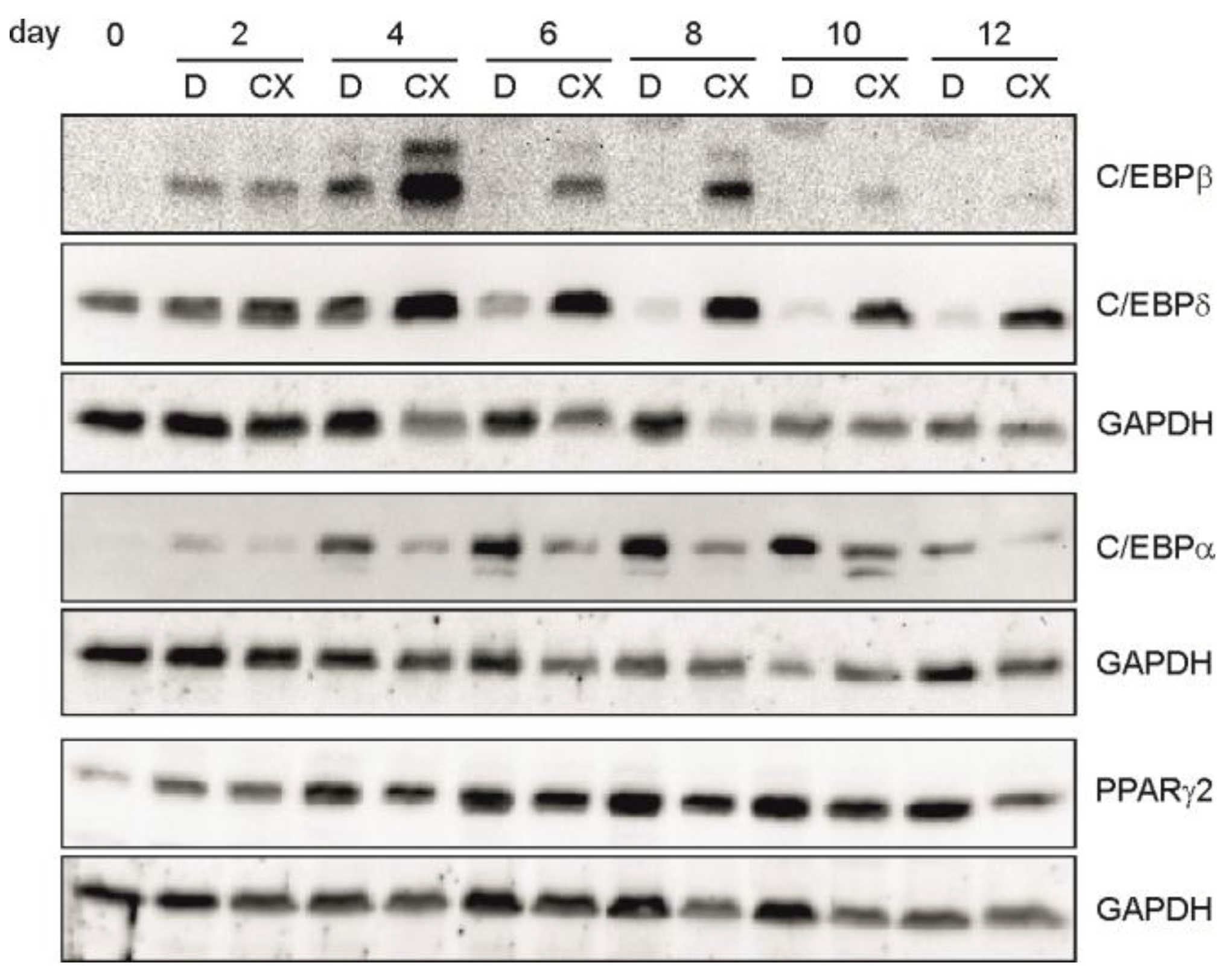
2.3. Inhibition of CK2 Activity with CX-4945 Influences Expression of Important Adipogenic Transcription Factors
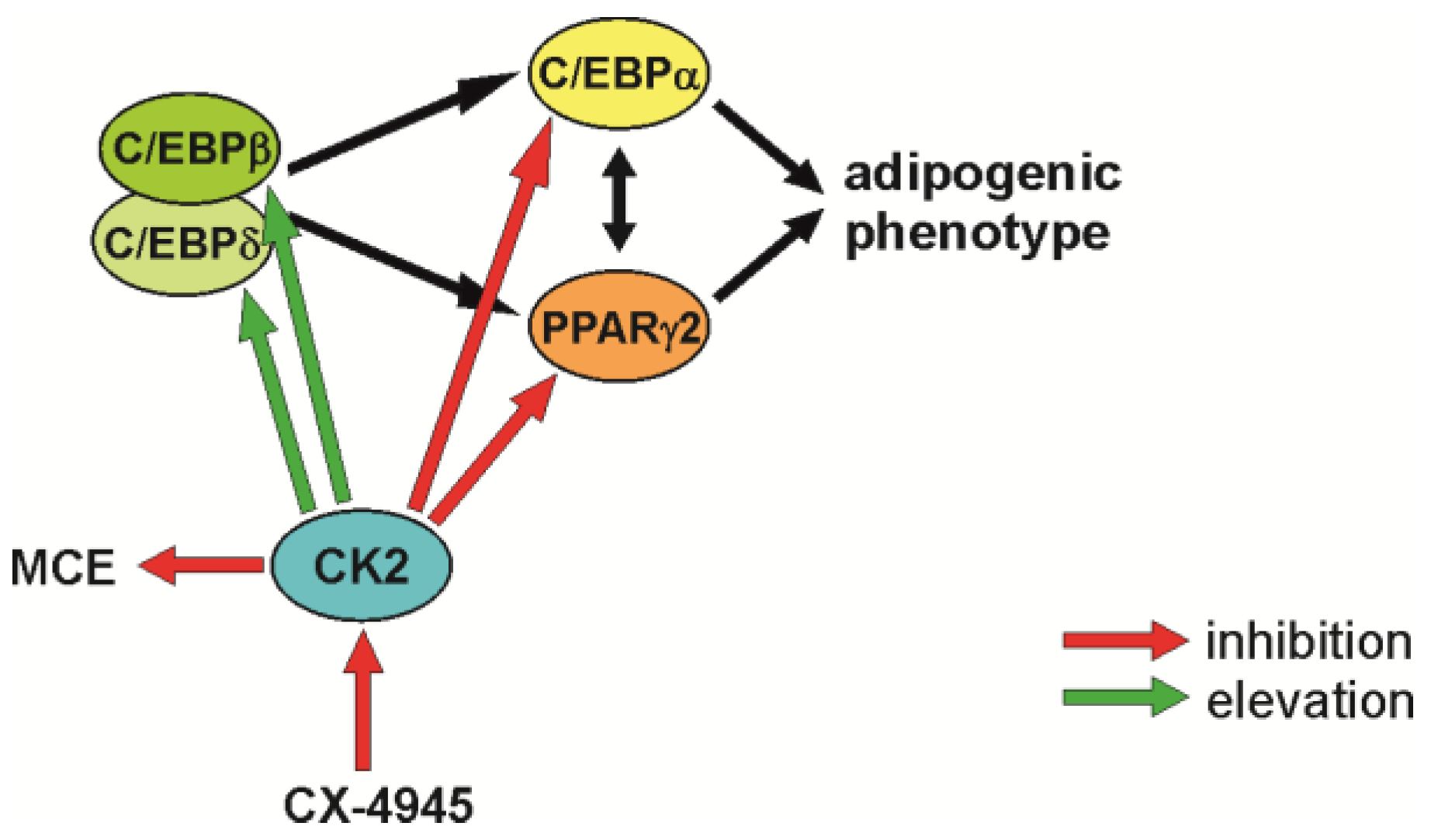
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Differentiation, and Treatment of Cells
4.2. Determination of Proliferation and Metabolic Activity
4.3. Extraction of Proteins
4.4. SDS–Polyacrylamide Gel Electrophoresis and Western Blot Analysis
4.5. CK2 In Vitro Phosphorylation Assay
4.6. Staining of Lipid Droplets in C3H/10T1/2 Cells
Author Contributions
Conflicts of Interest
References
- Cook, D.; Genever, P. Regulation of mesenchymal stem cell differentiation. Adv. Exp. Med. Biol. 2013, 786, 213–229. [Google Scholar]
- Guerra, B.; Issinger, O.G. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 1999, 20, 391–408. [Google Scholar] [CrossRef]
- Lou, D.Y.; Dominguez, I.; Toselli, P.; Landesman-Bollag, E.; O’Brien, C.; Seldin, D.C. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol. Cell. Biol. 2008, 28, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Mannowetz, N.; Kartarius, S.; Wennemuth, G.; Montenarh, M. Protein kinase CK2 and binding partners during spermatogenesis. Cell Mol. Life Sci. 2010, 67, 3905–3913. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.N.; Miller, P.J.; Hollenbach, A.D. Phosphorylation of serine 205 by the protein kinase CK2 persists on Pax3-FOXO1, but not Pax3, throughout early myogenic differentiation. Biochemistry 2009, 48, 11786–11795. [Google Scholar] [CrossRef] [PubMed]
- Filhol, O.; Cochet, C. Cellular functions of Protein kinase CK2: A dynamic affair. Cell Mol. Life Sci. 2009, 66, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Heriche, J.K.; Lebrin, F.; Rabilloud, T.; LeRoy, D.; Chambaz, E.M.; Goldberg, Y. Regulation of protein phosphatase 2A by direct interaction with casein kinase 2alpha. Science 1997, 276, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, B.; Litchfield, D.W. Biosynthesis of casein kinase II in lymphoid cell lines. Eur. J. Biochem. 1994, 220, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Siemer, S.; Boldyreff, B.; Issinger, O.G. Protein kinase CK2: Evidence for a protein kinase CK2 subunit fraction, devoid of the catalytic CK2 subunit, in mouse brain and testicles. FEBS Lett. 1999, 462, 353–357. [Google Scholar] [CrossRef]
- Stalter, G.; Siemer, S.; Becht, E.; Ziegler, M.; Remberger, K.; Issinger, O.-G. Asymmetric expression of protein kinase CK2 in human kidney tumors. Biochem. Biophys. Res. Commun. 1994, 202, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, I.; Degano, I.R.; Chea, K.; Cha, J.; Toselli, P.; Seldin, D.C. CK2alpha is essential for embryonic morphogenesis. Mol. Cell. Biochem. 2011, 356, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Buchou, T.; Vernet, M.; Blond, O.; Jensen, H.H.; Pointu, H.; Olsen, B.B.; Cochet, C.; Issinger, O.G.; Boldyreff, B. Disruption of the regulatory subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol. Cell. Biol. 2003, 23, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Toselli, P.A.; Russell, L.D.; Seldin, D.C. Globozoospermia in mice lacking the casein kinase II’ catalytic subunit. Nat. Genet. 1999, 23, 118–121. [Google Scholar] [PubMed]
- Schneider, H.R.; Reichert, G.H.; Issinger, O.G. Enhanced casein kinase II activity during mouse embryogenesis. Identification of a 110-kDa phosphoprotein as the major phosphorylation product in mouse embryos and Krebs II mouse ascites tumor cells. Eur. J. Biochem. 1986, 161, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; King, D.; Young, K.; Litchfield, D.W.; Petersen, N.O.; Nohe, A. Casein kinase 2 beta-subunit is a regulator of bone morphogenetic protein 2 signaling. Biophys. J. 2010, 99, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; Gurski, L.; Bonor, J.; Price, C.; Wang, L.; Beamer, W.G.; Nohe, A. Casein kinase 2 regulates in vivo bone formation through its interaction with bone morphogenetic protein receptor type Ia. Bone 2011, 49, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Moseychuk, O.; Akkiraju, H.; Dutta, J.; D’Angelo, A.; Bragdon, B.; Duncan, R.L.; Nohe, A. Inhibition of CK2 binding to BMPRIa induces C2C12 differentiation into osteoblasts and adipocytes. J. Cell Commun. Signal. 2013, 7, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Kumar, V.; Liu, H.; Youn, J.I.; Fishman, M.; Sherman, S.; Gabrilovich, D. Effects of notch signaling on regulation of myeloid cell differentiation in cancer. Cancer Res. 2014, 74, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Dovat, S.; Song, C.; Payne, K.J.; Li, Z. Ikaros, CK2 kinase, and the road to leukemia. Mol. Cell. Biochem. 2011, 356, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Gratton, M.O.; Torban, E.; Jasmin, S.B.; Theriault, F.M.; German, M.S.; Stifani, S. Hes6 promotes cortical neurogenesis and inhibits Hes1 transcription repression activity by multiple mechanisms. Mol. Cell. Biol. 2003, 23, 6922–6935. [Google Scholar] [CrossRef] [PubMed]
- Schwind, L.; Wilhelm, N.; Kartarius, S.; Montenarh, M.; Gorjup, E.; Götz, C. Protein kinase CK2 is necessary for the adipogenic differentiation of human mesenchymal stem cells. Biochem. Biophys. Acta 2014, 1853, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Cozza, G.; Bortolato, A.; Moro, S. How druggable is protein kinase CK2? Med. Res. Rev. 2009, 30, 419–462. [Google Scholar] [CrossRef] [PubMed]
- Cozza, G.; Pinna, L.A.; Moro, S. Protein kinase CK2 inhibitors: A patent review. Expert Opin. Ther. Pat. 2012, 22, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui-Jain, A.; Drygin, D.; Streiner, N.; Chua, P.; Pierre, F.; O’Brien, S.E.; Bliesath, J.; Omori, M.; Huser, N.; Ho, C.; et al. CX-4945, an Orally Bioavailable Selective Inhibitor of Protein Kinase CK2, Inhibits Prosurvival and Angiogenic Signaling and Exhibits Antitumor Efficacy. Cancer Res. 2010, 70, 10288–10298. [Google Scholar] [CrossRef] [PubMed]
- Battistutta, R.; Cozza, G.; Pierre, F.; Papinutto, E.; Lolli, G.; Sarno, S.; O’Brien, S.E.; Siddiqui-Jain, A.; Haddach, M.; Anderes, K.; et al. Unprecedented selectivity and structural determinants of a new class of protein kinase CK2 inhibitors in clinical trials for the treatment of cancer. Biochemistry 2011, 50, 8478–8488. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Chua, P.C.; O’Brien, S.E.; Siddiqui-Jain, A.; Bourbon, P.; Haddach, M.; Michaux, J.; Nagasawa, J.; Schwaebe, M.K.; Stefan, E.; et al. Pre-clinical characterization of CX-4945, a potent and selective small molecule inhibitor of CK2 for the treatment of cancer. Mol. Cell Biochem. 2011, 356, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.H. Druggability of the CK2 inhibitor CX-4945 as an anticancer drug and beyond. Arch. Pharm. Res. 2012, 35, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Reznikoff, C.A.; Brankow, D.W.; Heidelberger, C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973, 33, 3231–3238. [Google Scholar] [PubMed]
- Kuenzel, E.A.; Krebs, E.G. A synthetic peptide substrate specific for casein kinase II. Proc. Natl. Acad. Sci. USA 1985, 82, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [PubMed]
- Pierre, F.; Chua, P.C.; O’Brien, S.E.; Siddiqui-Jain, A.; Bourbon, P.; Haddach, M.; Michaux, J.; Nagasawa, J.; Schwaebe, M.K.; Stefan, E.; et al. Discovery and SAR of 5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer. J. Med. Chem. 2011, 54, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.J.; Bae, K.J.; Lee, Y.; Kim, J. The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies. Front. Pharmacol. 2015, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Janderova, L.; McNeil, M.; Murrell, A.N.; Mynatt, R.L.; Smith, S.R. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes. Res. 2003, 11, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Sarraf, P.; Troy, A.E.; Bradwin, G.; Moore, K.; Milstone, D.S.; Spiegelman, B.M.; Mortensen, R.M. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 1999, 4, 611–617. [Google Scholar] [CrossRef]
- Ren, D.; Collingwood, T.N.; Rebar, E.J.; Wolffe, A.P.; Camp, H.S. PPARγ knockdown by engineered transcription factors: Exogenous PPARγ2 but not PPARγ1 reactivates adipogenesis. Genes Dev. 2002, 16, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Nohe, A. CK2.1, a novel peptide, induces articular cartilage formation in vivo. J. Orthop. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mestres, P.; Boldyreff, B.; Ebensperger, C.; Hameister, H.; Issinger, O.-G. Expression of casein kinase 2 during mouse embryogenesis. Acta Anat. 1994, 149, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Maridor, G.; Park, W.; Krek, W.; Nigg, E.A. Casein kinase II. cDNA sequences, developmental expression, and tissue distribution of mRNAs for alpha, alpha’, and beta subunits of the chicken enzyme. J. Biol. Chem. 1991, 266, 2362–2368. [Google Scholar] [PubMed]
- Hu, E.; Rubin, C.S. Casein kinase II from Caenorhabditis elegans. Cloning, characterization, and developmental regulation of the gene encoding the beta subunit. J. Biol. Chem. 1991, 266, 19796–19802. [Google Scholar] [PubMed]
- Perez, M.; Grande, J.; Itarte, E. Developmental changes in rat hepatic casein kinases 1 and 2. Eur. J. Biochem. 1987, 170, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, N.; Kostelnik, K.; Götz, C.; Montenarh, M. Protein kinase CK2 is implicated in early steps of the differentiation of preadipocytes into adipocytes. Mol. Cell Biochem. 2012, 365, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Prowald, K.; Fischer, H.; Issinger, O.G. Enhanced casein kinase II activity in human tumour cell cultures. FEBS Lett. 1984, 176, 479–483. [Google Scholar] [CrossRef]
- Faust, R.A.; Gapany, M.; Tristani, P.; Davis, A.; Adams, G.L.; Ahmed, K. Elevated protein kinase CK2 activity in chromatin of head and neck tumors: Association with malignant transformation. Cancer Lett. 1996, 101, 31–35. [Google Scholar] [CrossRef]
- Wu, Z.; Bucher, N.L.; Farmer, S.R. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol. Cell. Biol. 1996, 16, 4128–4136. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.T.; Lane, M.D. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 1992, 6, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Schwind, L.; Zimmer, A.; Götz, C.; Montenarh, M. CK2 phosphorylation of C/EBP regulates its transcription factor activity. Int. J. Biochem. Cell Biol. 2015, 61, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T 4. Nature 1970, 227, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Faust, M.; Schuster, N.; Montenarh, M. Specific binding of protein kinase CK2 catalytic subunits to tubulin. FEBS Lett. 1999, 462, 51–56. [Google Scholar] [CrossRef]
- Nastainczyk, W.; Schmidt-Spaniol, I.; Boldyreff, B.; Issinger, O.-G. Isolation and characterization of a monoclonal anti-protein kinase CK2 -subunit antibody of the IgG class for the direct detection of CK2-subunit in tissue cultures of various mammalian species and human tumors. Hybridoma 1995, 14, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Nastainczyk, W.; Issinger, O.G.; Guerra, B. Epitope analysis of the MAb 1AD9 antibody detection site in human protein kinase CK2alpha-subunit. Hybrid. Hybridomics 2003, 22, 87–90. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwind, L.; Schetting, S.; Montenarh, M. Inhibition of Protein Kinase CK2 Prevents Adipogenic Differentiation of Mesenchymal Stem Cells Like C3H/10T1/2 Cells. Pharmaceuticals 2017, 10, 22. https://doi.org/10.3390/ph10010022
Schwind L, Schetting S, Montenarh M. Inhibition of Protein Kinase CK2 Prevents Adipogenic Differentiation of Mesenchymal Stem Cells Like C3H/10T1/2 Cells. Pharmaceuticals. 2017; 10(1):22. https://doi.org/10.3390/ph10010022
Chicago/Turabian StyleSchwind, Lisa, Sarah Schetting, and Mathias Montenarh. 2017. "Inhibition of Protein Kinase CK2 Prevents Adipogenic Differentiation of Mesenchymal Stem Cells Like C3H/10T1/2 Cells" Pharmaceuticals 10, no. 1: 22. https://doi.org/10.3390/ph10010022




