In Vitro and In Vivo Studies of Alar-Nasal Cartilage Using Autologous Micro-Grafts: The Use of the Rigenera® Protocol in the Treatment of an Osteochondral Lesion of the Nose
Abstract
:1. Introduction
2. Results
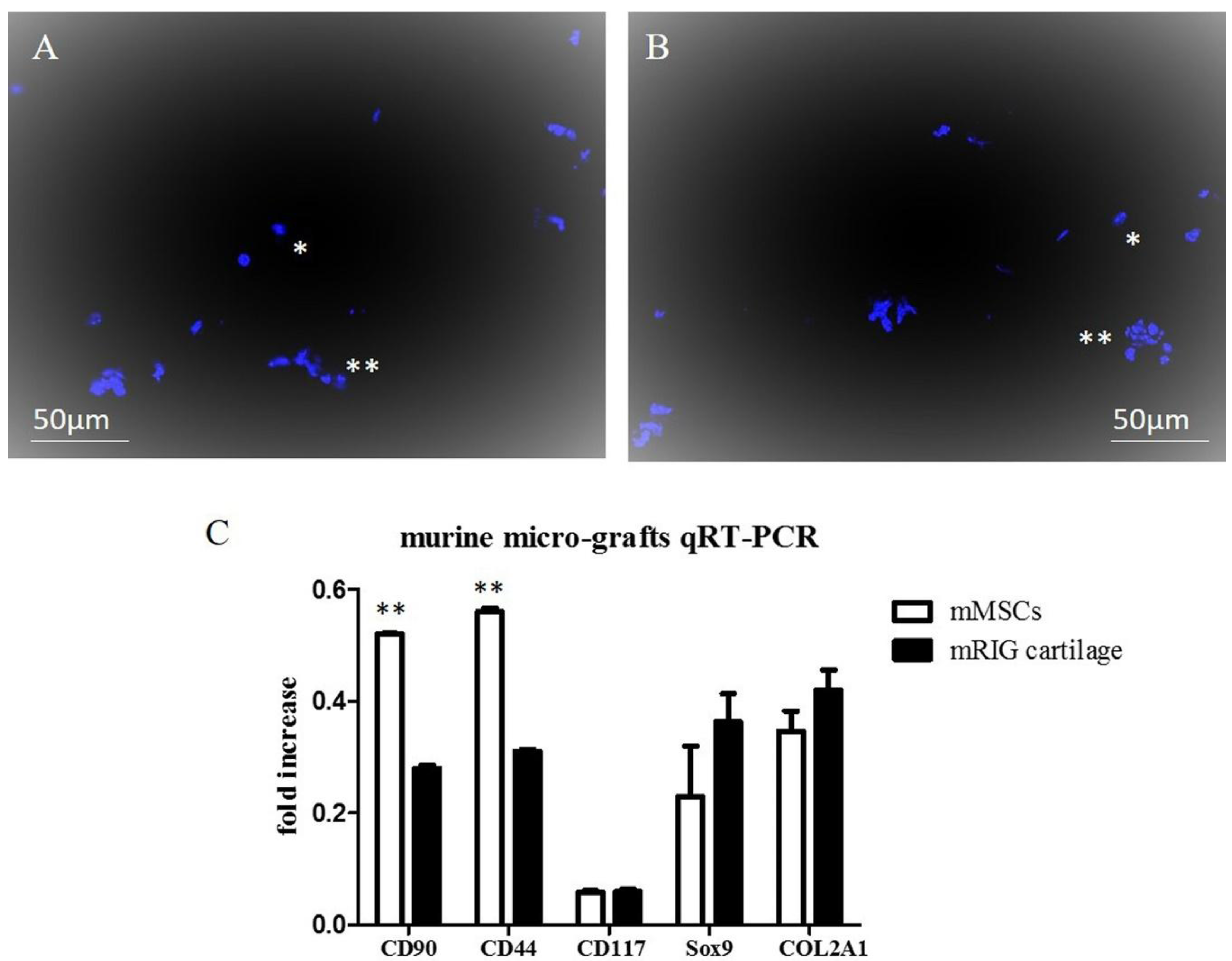
2.1. Chondrocyte Murine Micro-Grafts Analysis
2.2. Chondrocyte Human Micro-Grafts Analysis
2.3. In Vivo Micro-Grafts
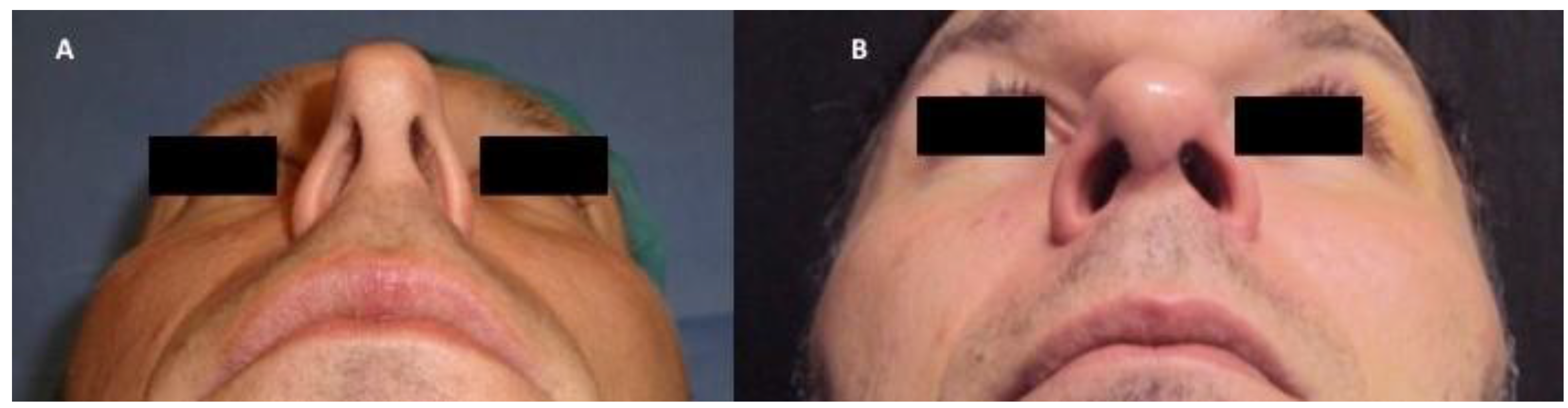
2.4. Patients and Clinical Procedure
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Analysis of Micro-Grafts from Auricular Murine Cartilage
4.3. Analysis of Micro-Grafts from Auricular Human Cartilage
4.4. Chondrocyte Micro-Grafts Preparation for in Vivo Treatment
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chan, V.O.; Moran, D.E.; Mwangi, I.; Eustace, S.J. Prevalence and clinical significance of chondromalacia isolated to the anterior margin of the lateral femoral condyle as a component of patellofemoral disease: Observations at MR imaging. Skelet. Radiol. 2013, 42, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.J.; Ho, J.; Allen, C.R. Evaluation and management of osteochondral lesions of the knee. Phys. Sportsmed. 2011, 39, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, W.; Wang, X.; Pan, F.; Liu, Z.; Halliday, A.; Jin, X.; Antony, B.; Cicuttini, F.; Jones, G.; et al. Mass effect and signal intensity alteration in the suprapatellar fat pad: Associations with knee symptoms and structure. Osteoarthr. Cartil. 2014, 22, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Lankhorst, N.E.; Damen, J.; Oei, E.H.; Verhaar, J.A.N.; Bierma-Zeinstra, S.M.A.; van Middelkoop, M. Incidence, prevalence, natural course and prognosis of patellofemoral osteoarthritis; data of cohort hip and cohort knee study. Osteoarthr. Cartil. 2015, 23, A42–A43. [Google Scholar] [CrossRef]
- Anandacoomarasamy, A.; Smith, G.; Leibman, S.; Caterson, I.; Giuffre, B.; Fransen, M.; Sambrook, P.; March, L. Cartilage defects are associated with physical disability in obese adults. Rheumatology 2009, 48, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, W.H.; Liu, S.W. Comparative Study of Functional Nasal Reconstruction Using Structural Reinforcement. JAMA Facial Plast. Surg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, G.; Scopelliti, D. Nasal Valve Collapse: Our Treatment Protocol. J. Craniofac. Surg. 2017, 28, e359–e360. [Google Scholar] [CrossRef] [PubMed]
- Goudakos, J.K.; Fishman, J.M.; Patel, K. A systematic review of the surgical techniques for the treatment of internal nasal valve collapse: Where do we stand? Clin. Otolaryngol. 2017, 42, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Papel, I.D. Spreader Grafts in Functional Rhinoplasty. Facial Plast. Surg. 2016, 32, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Monllau, J.C.; Leal, J.; Voss, C.; Pelfort, X.; Tey, M.; Pavlovich, R.I. Good outcome after meniscal repair using an all-inside suturing system in combination with high-frequency biostimulation. Orthopedics 2010, 33, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, P.; Giavaresi, G.; Fini, M.; Guzzardella, G.A.; Morrone, G.; Carpi, A.; Giardino, R. Laser biostimulation of cartilage: In vitro evaluation. Biomed. Pharmacother. 2001, 55, 117–120. [Google Scholar] [CrossRef]
- Macmull, S.; Jaiswal, P.K.; Bentley, G.; Skinner, J.A.; Carrington, R.W.J.; Briggs, T.W.R. The role of autologous chondrocyte implantation in the treatment of symptomatic chondromalacia patellae. Int. Orthop. 2012, 36, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Marmotti, A.; Rossi, R.; Castoldi, F.; Roveda, E.; Michielon, G.; Peretti, G.M. PRP and articular cartilage: A clinical update. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Goodell, B.M.A.; Brose, K.; Paradis, G.; Conner, A.S.; Mulligan, R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996, 183, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Romano, M.; Zarantonello, L.; Ruffolo, C.; Neri, D.; Bassi, N.; Giordano, A.; Zanus, G.; Ferraro, G.A.; Cillo, U. The role of adipose stem cells in inflammatory bowel disease: From biology to novel therapeutic strategies. Cancer Biol. Ther. 2016, 17, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, J.; Zhang, X.; Liu, Y.; Chen, J.; Hu, B.; Song, J.; Zhang, Y. Strategies to optimize adult stem cell therapy for tissue regeneration. Int. J. Mol. Sci. 2016, 17, 982. [Google Scholar] [CrossRef] [PubMed]
- Mattioli-Belmonte, M.; Teti, G.; Salvatore, V.; Focaroli, S.; Orciani, M.; Dicarlo, M.; Fini, M.; Orsini, G.; Di Primio, R.; Falconi, M. Stem cell origin differently affects bone tissue engineering strategies. Front. Physiol. 2015, 6, 266. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Mitchell, K.; Soans, J.; Kim, L.; Zaidi, R. The use of mesenchymal stem cells for cartilage repair and regeneration: A systematic review. J. Orthop. Surg. Res. 2017, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Trovato, L.; Monti, M.; del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A New Medical Device Regeneracons® Allows to Obtain Viable Micro-Grafts from Mechanical Disaggregation of Human Tissues. J. Cell. Physiol. 2015, 230, 2299–2303. [Google Scholar] [CrossRef] [PubMed]
- Townsend-Nicholson, A.; Jayasinghe, S.N. Cell electrospinning: A unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules 2006, 7, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N. Cell electrospinning: A novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst 2013, 138, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Purpura, V.; Bondioli, E.; Graziano, A.; Trovato, L.; Melandri, D.; Ghetti, M.; Marchesini, A.; Cusella De Angelis, M.G.; Benedetti, L.; Ceccarelli, G.; et al. Tissue Characterization after a New Disaggregation Method for Skin Micro-Grafts Generation. J. Vis. Exp. 2016, 4, e53579. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, M.; Brunetti, M.; Camandona, M.; Trovato, L.; Graziano, A. A new medical device, based on Rigenera® protocol, in the management of complex wounds. J. Stem Cells Res. Rev. Rep. 2014, 1, 3. [Google Scholar]
- Marcarelli, M.; Trovato, L.; Novarese, E.; Riccio, M.; Graziano, A. Rigenera® protocol in the treatment of surgical wound dehiscence. Int. Wound J. 2016, 14, 277–281. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Graziano, A.; Trovato, L.; Ceccarelli, G.; Romano, M.; Marcarelli, M.; Cusella De Angelis, G.M.; Cillo, U.; Riccio, M.; Ferraro, G.A. A Regenerative Approach with Dermal Micrografts in the Treatment of Chronic Ulcers. Stem Cell Rev. 2017, 13, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.; Carinci, F.; Scolaro, S.; D’Aquino, R. Periodontal tissue generation using autologous dental ligament micro-grafts: Case report with 6 months follow-up. Ann. Oral. Maxillofac. Surg. 2013, 1, 20. [Google Scholar] [CrossRef]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef] [PubMed]
- De Crombrugghe, B.; Lefebvre, V.; Behringer, R.R.; Bi, W.; Murakami, S.; Huang, W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000, 19, 389–394. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. A combined use of Chondrocytes Micro Grafts (CMG) Mixed with Platelet Rich Plasma (PRP) in Patients Affected by Pinch Nose Deformity. J. Regen. Med. 2016, 5. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, MG.; Bielli, A.; Orlandi, A.; Cervelli, V. Reconstruction of Alar Nasal Cartilage Defects Using a Tissue Engineering Technique Based on a Combined Use of Autologous Chondrocyte Micrografts and Platelet-rich Plasma: Preliminary Clinical and Instrumental Evaluation. Plast. Reconstr. Surg. Glob. Open 2016, 26, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Z.; Ma, N.; Hao, C.; Guo, W.; Zou, G.; Zhang, Y.; Chen, M.; Gao, S.; Peng, J.; et al. Advances and Prospects in Stem Cells for Cartilage Regeneration. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Eo, S.H.; Abbas, Q.; Ahmed, M.; Kim, S.J. Applications of Chondrocyte-Based Cartilage Engineering: An Overview. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Bouffi, C.; Merceron, C.; Gordeladze, J.; Brondello, J.M.; Jorgensen, C.; Weiss, P.; Guicheux, J.; Noël, D. Cartilage tissue engineering: Towards a biomaterial-assisted mesenchymal stem cell therapy. Curr. Stem Cell Res. Ther. 2009, 4, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, R.; Trovato, L.; Graziano, A.; Ceccarelli, G.; de Angelis, G.C.; Marangini, A.; Nisio, A.; Galli, M.; Pasi, M.; Finotti, M.; et al. Periosteum-derived micro-grafts for tissue regeneration of human maxillary bone. J. Transl. Sci. 2016. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Cometa, A.; Villa, R.; Novara, F.; Moretta, A.; Avanzini, A.; Maccario, R.; Daidone, M.G.; Zaffaroni, N.; Zuffardi, O.; et al. Human bone marrow-derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007, 67, 9142–9149. [Google Scholar] [CrossRef] [PubMed]
- Prè, D.; Ceccarelli, G.; Gastaldi, G.; Asti, A.; Saino, E.; Visai, L.; Benazzo, F.; Cusella De Angelis, M.G.; Magenes, G. The differentiation of human adipose-derived stem cells (hASCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment. Bone 2011, 49, 295–303. [Google Scholar] [CrossRef] [PubMed]


| Genes | Forward Sequences | Reverse Sequences |
|---|---|---|
| mCD90 | 5′ AAG TCG GAA CTC TTG GCA CC 3′ | 5′ CCA GGC GAA GGT TTT GGT TC 3′ |
| mCD44 | 5′ CGA ACC AGG ACA GTG GAG TG 3′ | 5′ TCT GCC CAC ACC TTC TCC TAC TAT 3′ |
| mCD117 | 5′ TGA ACG GTA ACA TGG CTG CAT T 3′ | 5′ ACC ACC GTA AAT GTG TCC CC 3′ |
| mSOX-9 | 5′ AGA CTC ACA TCT CTC CTA ATG CT 3′ | 5′ ACG TCG GTT TTG GGA GTG G 3′ |
| mCOL2A1 | 5′ GGC TCC CAA CAC CGC TAA C 3′ | 5′ GAT GTT CTG GGA GCC CTC AGT 3′ |
| *mPGK | 5′ CAA AAT GTC GCT TTC CAA CAA G 3′ | 5′ AAC GTT GAA GTC CAC CCT CAT C 3′ |
| hCD90 | 5′ CAG CGG AAG ACC CCA GT 3′ | 5′ CGT TAG GCT GGT CAC CTT CT 3′ |
| hCD44 | 5′ TTA CAG CCT CAG CAG AGC AC 3′ | 5′ TGA CCT AAG ACG GAG GGA GG 3′ |
| hCD117 | 5′ GCA CAA TGG CAC GGT TGA AT 3′ | 5′ GGT GTG GGG ATG GAT TTG CT 3′ |
| hSOX-9 | 5′ AGG AGA ACC CCA AGA TGC AC 3′ | 5′ GAG GCG TTT TGC TTC GTC AA 3′ |
| hCOL2A1 | 5′ AGG ACT GAC CAA GAT GGG AA 3′ | 5′ AGG GGA GCT GGC TAC TTC TC 3′ |
| *hGAPDH | 5′ AGC CTC AAG ATC ATC AGC AAT GCC 3′ | 5′ TGT GGT CAT GAG TCC TTC CAC GAT 3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, G.; Gentile, P.; Marcarelli, M.; Balli, M.; Ronzoni, F.L.; Benedetti, L.; Cusella De Angelis, M.G. In Vitro and In Vivo Studies of Alar-Nasal Cartilage Using Autologous Micro-Grafts: The Use of the Rigenera® Protocol in the Treatment of an Osteochondral Lesion of the Nose. Pharmaceuticals 2017, 10, 53. https://doi.org/10.3390/ph10020053
Ceccarelli G, Gentile P, Marcarelli M, Balli M, Ronzoni FL, Benedetti L, Cusella De Angelis MG. In Vitro and In Vivo Studies of Alar-Nasal Cartilage Using Autologous Micro-Grafts: The Use of the Rigenera® Protocol in the Treatment of an Osteochondral Lesion of the Nose. Pharmaceuticals. 2017; 10(2):53. https://doi.org/10.3390/ph10020053
Chicago/Turabian StyleCeccarelli, Gabriele, Pietro Gentile, Marco Marcarelli, Martina Balli, Flavio Lorenzo Ronzoni, Laura Benedetti, and Maria Gabriella Cusella De Angelis. 2017. "In Vitro and In Vivo Studies of Alar-Nasal Cartilage Using Autologous Micro-Grafts: The Use of the Rigenera® Protocol in the Treatment of an Osteochondral Lesion of the Nose" Pharmaceuticals 10, no. 2: 53. https://doi.org/10.3390/ph10020053







