A Short-Term Biological Indicator for Long-Term Kidney Damage after Radionuclide Therapy in Mice
Abstract
:1. Introduction
2. Results and Discussion
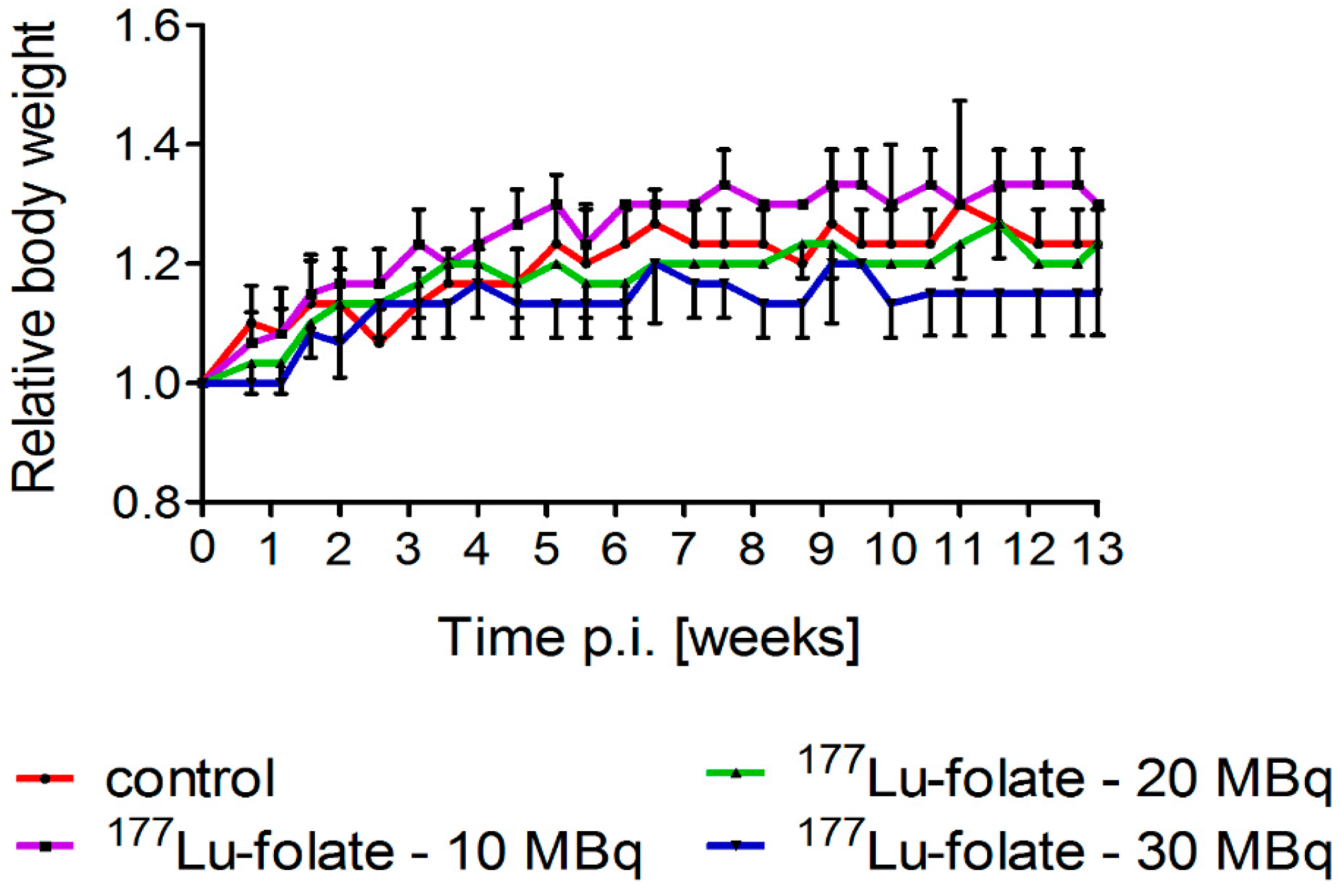
2.1. Effect of 177Lu-Folate Treatment on Body Weights of Mice
2.2. Effect of 177Lu-Folate Treatment on the Levels of Urine and Plasma Biomarkers
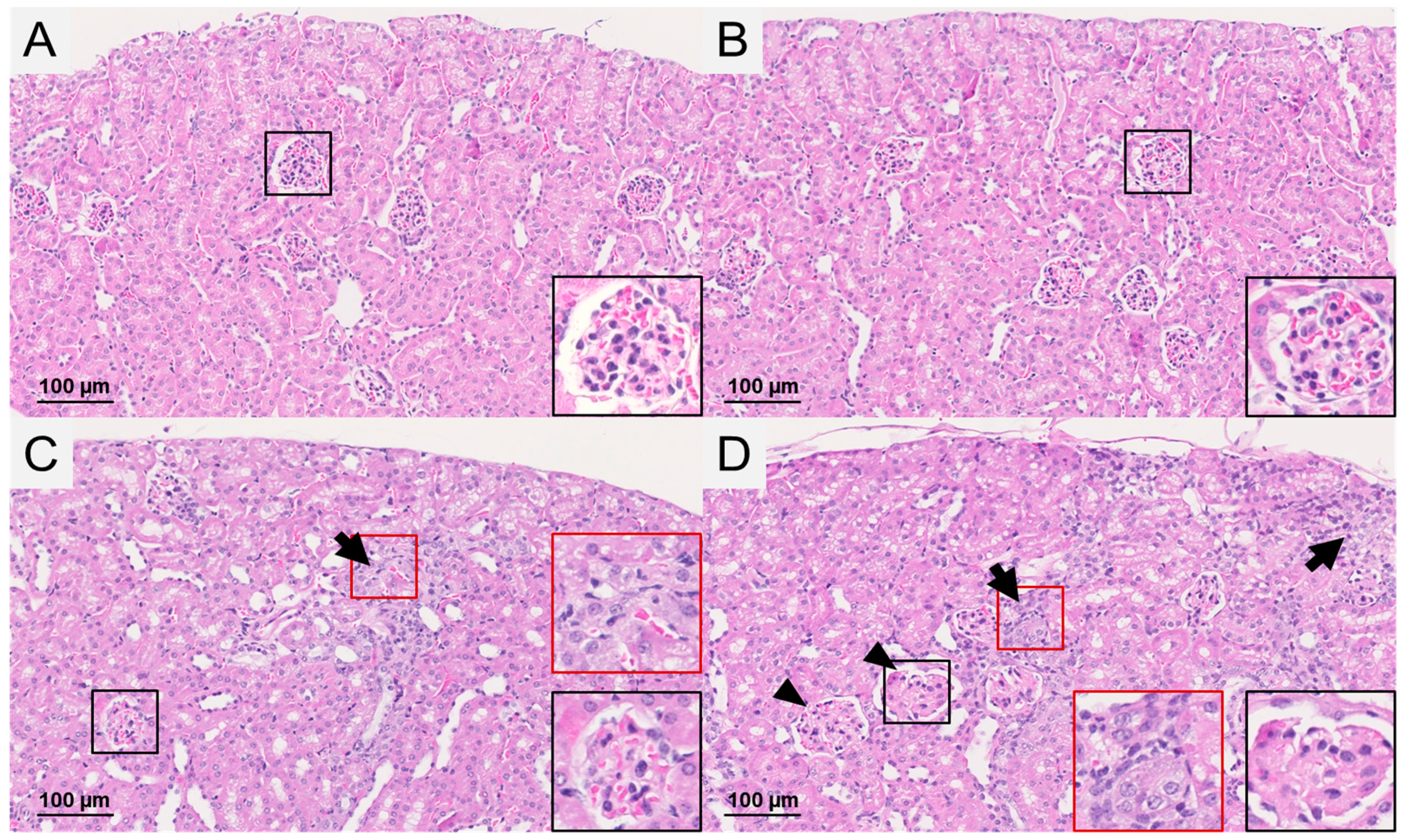
2.3. Renal Weight and Histopathological Observations
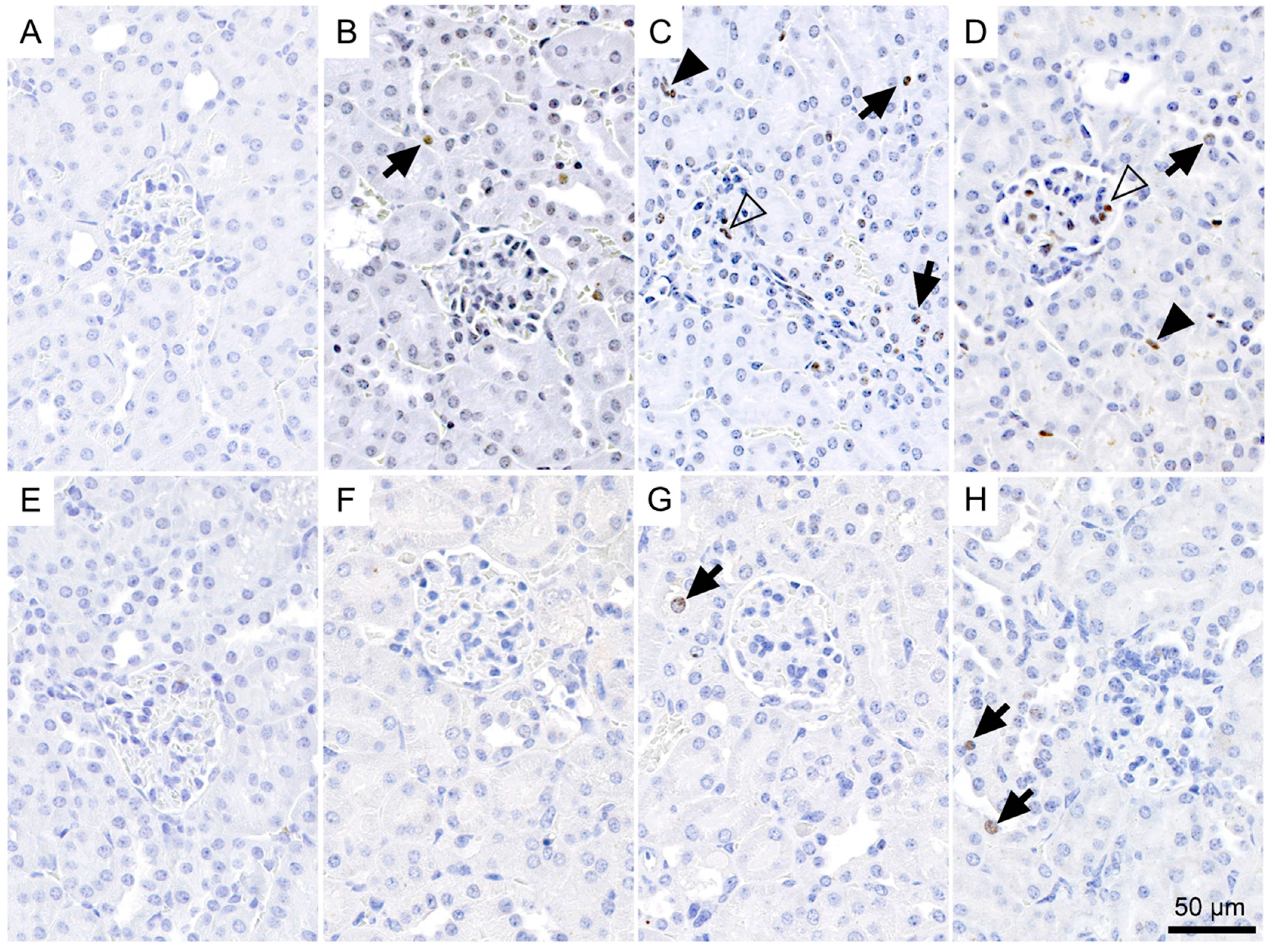
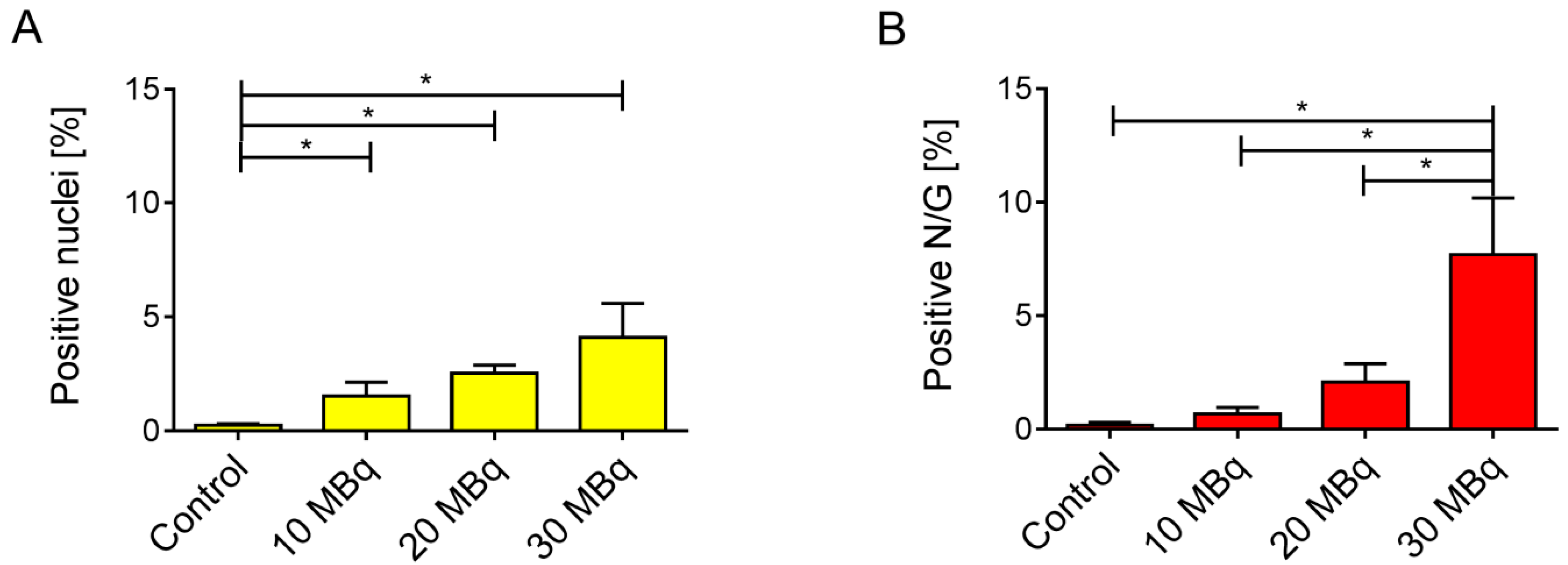
2.4. Immunohistological Detection of DNA Double-Strand Breaks
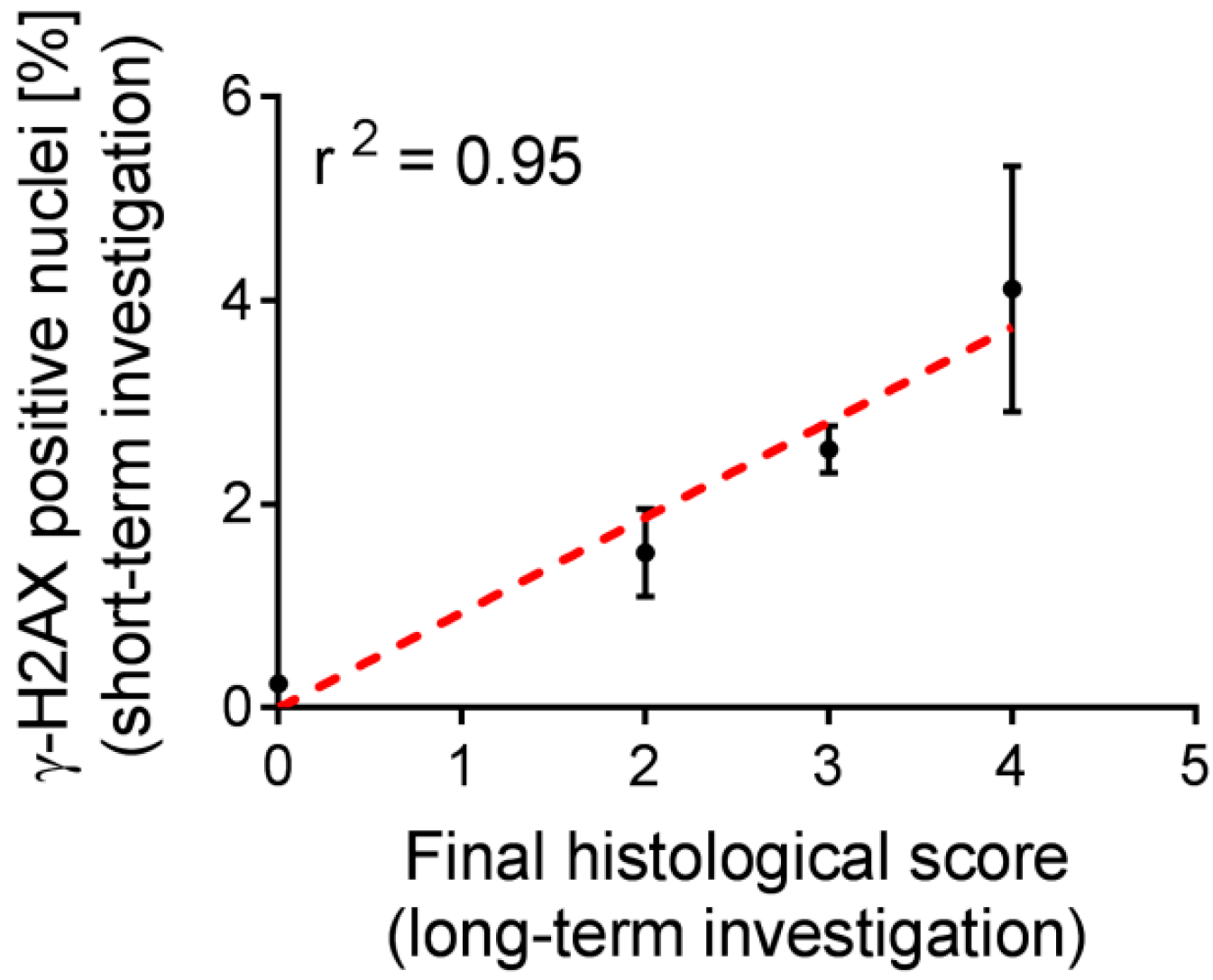
2.5. Correlation of Biological Markers with a Long-Term Kidney Damage
3. Conclusions
4. Materials and Methods
4.1. Preparation of 177Lu-folate
4.2. Animal Experiments
4.3. Determination of Plasma and Urinary Markers
4.4. Necropsy, Tissue Processing and Histological Examination
4.5. Immunohistochemistry for the Detection of DNA Double-Strand Breaks
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gudkov, S.V.; Shilyagina, N.Y.; Vodeneev, V.A.; Zvyagin, A.V. Targeted radionuclide therapy of human tumors. Int. J. Mol. Sci. 2015, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.; Cybulla, M.; Weiner, S.M.; Van De Wiele, C.; Ham, H.; Dierckx, R.A.; Otte, A. Renal toxicity after radionuclide therapy. Radiat. Res. 2004, 161, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.E.; Bonsib, S.M. Radiation nephropathy: A review. Scanning Microsc. 1995, 9, 535–560. [Google Scholar] [PubMed]
- Cohen, E.P.; Robbins, M.E. Radiation nephropathy. Semin. Nephrol. 2003, 23, 486–499. [Google Scholar] [CrossRef]
- Moll, S.; Nickeleit, V.; Müller-Brand, J.; Brunner, F.P.; Maecke, H.R.; Mihatsch, M.J. A new cause of renal thrombotic microangiopathy: 9 Y-dotatoc internal radiotherapy. Am. J. Kidney Dis. 2001, 37, 847–851. [Google Scholar] [CrossRef]
- Müller, C.; Vlahov, I.R.; Santhapuram, H.K.; Leamon, C.P.; Schibli, R. Tumor targeting using 67Ga-DOTA-Bz-folate - investigations of methods to improve the tissue distribution of radiofolates. Nucl. Med. Biol. 2011, 38, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Rolleman, E.J.; Melis, M.; Valkema, R.; Boerman, O.C.; Krenning, E.P.; de Jong, M. Kidney protection during peptide receptor radionuclide therapy with somatostatin analogues. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Kouvaris, J.R.; Kouloulias, V.E.; Vlahos, L.J. Amifostine: The first selective-target and broad-spectrum radioprotector. Oncologist 2007, 12, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Rolleman, E.J.; Forrer, F.; Bernard, B.; Bijster, M.; Vermeij, M.; Valkema, R.; Krenning, E.P.; de Jong, M. Amifostine protects rat kidneys during peptide receptor radionuclide therapy with [177Lu-DOTA°,Tyr3]octreotate. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I. Radiation damage and radioprotectants: New concepts in the era of molecular medicine. Br. J. Radiol. 2012, 85, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Schibli, R. Prospects in folate receptor-targeted radionuclide therapy. Front. Oncol. 2013, 3, 249. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Reddy, J.A.; Leamon, C.P.; Schibli, R. Effects of the antifolates pemetrexed and CB3717 on the tissue distribution of 99mTc-EC20 in xenografted and syngeneic tumor-bearing mice. Mol. Pharm. 2010, 7, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Pellegrini, G.; Vermeulen, C.; van der Meulen, N.P.; Köster, U.; Bernhardt, P.; Schibli, R.; Müller, C. Contribution of auger/conversion electrons to renal side effects after radionuclide therapy: Preclinical comparison of 161Tb-folate and 177Lu-folate. EJNMMI Res. 2016, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Pouliliou, S.; Koukourakis, M.I. Gamma histone 2AX (γ-H2AX)as a predictive tool in radiation oncology. Biomarkers 2014, 19, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Vegt, E.; de Jong, M.; Wetzels, J.F.; Masereeuw, R.; Melis, M.; Oyen, W.J.; Gotthardt, M.; Boerman, O.C. Renal toxicity of radiolabeled peptides and antibody fragments: Mechanisms, impact on radionuclide therapy, and strategies for prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Molne, J.; Forssell-Aronsson, E.; Konijnenberg, M.; Bernhardt, P. Nephrotoxicity profiles and threshold dose values for [177Lu]-DOTATATE in nude mice. Nucl. Med. Biol. 2012, 39, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Erbas, B.; Tuncel, M. Renal function assessment during peptide receptor radionuclide therapy. Semin. Nucl. Med. 2016, 46, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Struthers, H.; Winiger, C.; Zhernosekov, K.; Schibli, R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 2013, 54, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Reber, J.; Brandt, S.; Bernhardt, P.; Groehn, V.; Schibli, R.; Müller, C. Folate receptor-targeted radionuclide therapy: Preclinical investigation of anti-tumor effects and potential radionephropathy. Nucl. Med. Biol. 2015, 42, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P. Biomarkers for the early detection of acute kidney injury. Curr. Opin. Pediatr. 2011, 23, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, C.L. Biomarkers of acute kidney injury. Adv. Chronic Kidney Dis. 2008, 15, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Herget-Rosenthal, S.; van Wijk, J.A.; Brocker-Preuss, M.; Bokenkamp, A. Increased urinary cystatin c reflects structural and functional renal tubular impairment independent of glomerular filtration rate. Clin. Biochem. 2007, 40, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Koyner, J.L.; Bennett, M.R.; Worcester, E.M.; Ma, Q.; Raman, J.; Jeevanandam, V.; Kasza, K.E.; O’Connor, M.F.; Konczal, D.J.; Trevino, S.; et al. Urinary cystatin c as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008, 74, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Faga, T.; Riccio, E.; Sabbatini, M.; Pisani, A.; Michael, A. The potential use of biomarkers in predicting contrast-induced acute kidney injury. Int. J. Nephrol. Renovasc. Dis. 2016, 9, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Halligan, B.D.; Wakim, B.T.; Savin, V.J.; Cohen, E.P.; Moulder, J.E. The urine proteome as a biomarker of radiation injury. Proteom. Clin. Appl. 2008, 2, 1065–1086. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.K.; Maturen, K.E.; Feng, M.U.; Wizauer, E.J.; Watcharotone, K.; Parker, R.A.; Ellis, J.H. Renal remodeling after abdominal radiation therapy: Parenchymal and functional changes. Am. J. Roentgenol. 2014, 203, W192–W198. [Google Scholar] [CrossRef] [PubMed]
- Hubenak, J.R.; Zhang, Q.; Branch, C.D.; Kronowitz, S.J. Mechanisms of injury to normal tissue after radiotherapy: A review. Plast. Reconstr. Surg. 2014, 133, 49e–56e. [Google Scholar] [CrossRef] [PubMed]
- Mahrhofer, H.; Bürger, S.; Oppitz, U.; Flentje, M.; Djuzenova, C.S. Radiation induced DNA damage and damage repair in human tumor and fibroblast cell lines assessed by histone H2AX phosphorylation. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Vallis, K.A.; Reilly, R.M. Computational analysis of the number, area and density of γH2AX foci in breast cancer cells exposed to 111In-DTPA-hEGF or γ-rays using image-j software. Int. J. Raidat. Biol. 2009, 85, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Ivashkevich, A.; Redon, C.E.; Nakamura, A.J.; Martin, R.F.; Martin, O.A. Use of the g-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012, 327, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Redon, C.E.; Nakamura, A.J.; Zhang, Y.W.; Ji, J.J.; Bonner, W.M.; Kinders, R.J.; Parchment, R.E.; Doroshow, J.H.; Pommier, Y. Histone γH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clin. Cancer Res. 2010, 16, 4532–4542. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, B.; Vezhenkova, I.; Firsanov, D.; Solovjeva, L.; Svetlova, M.; Mikhailov, V.; Tomilin, N. Slow elimination of phosphorylated histone γ-H2AX from DNA of terminally differentiated mouse heart cells in situ. Biochem. Biophys. Res. Commun. 2006, 347, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Barnard, S.; Rothkamm, K. Gamma-H2AX-based dose estimation for whole and partial body radiation exposure. PLoS ONE 2011, 6, e25113. [Google Scholar] [CrossRef] [PubMed]
- Urbschat, A.; Obermuller, N.; Haferkamp, A. Biomarkers of kidney injury. Biomarkers 2011, 16 (Suppl. 1), S22–S30. [Google Scholar] [CrossRef] [PubMed]
- Redon, C.; Pilch, D.; Rogakou, E.; Sedelnikova, O.; Newrock, K.; Bonner, W. Histone H2A variants H2AX and H2AZ. Curr. Opin. Genet. Dev. 2002, 12, 162–169. [Google Scholar] [CrossRef]
| 177Lu-folate (MBq) | Time of Euthanasia | Score Glomeruli (Average) | Score Tubules (Average) | Score Interstitium (Average) | Cumulative Score | Final Score * |
|---|---|---|---|---|---|---|
| Results of the present study | ||||||
| - | 2 weeks | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | |
| 20 | 0 | 0 | 0 | 0 | 0 | |
| 30 | 0 | 0 | 0 | 0 | 0 | |
| - | 3 months | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | |
| 20 | 0 | 0.3 | 0 | 0.3 | 0 (n = 2); 1 (n = 1) | |
| 30 | 2.0 | 1.5 | 0 | 3.5 | 2 | |
| Results of a previous study reported by Haller et al. [15] | ||||||
| - | 8 months | 0 | 0 | 0 | 0 | 0 |
| 10 | 2.0 | 1.5 | 0.4 | 3.9 | 2 | |
| 20 | 3.8 | 3.3 | 3.3 | 10.4 | 3 | |
| 30 | 5–6 months ** | 5 | 4.2 | 4.6 | 13.8 | 4 |
| Saline | 177Lu-folate | |||
|---|---|---|---|---|
| Injected activity (MBq) | - | 10 | 20 | 30 |
| Kidney dose (Gy) | - | ~23 | ~46 | ~69 |
| Cumulative Score (Glomeruli, Tubules, Interstitium) | Final Score | Renal Injury |
|---|---|---|
| 0–0.9 | 0 | no histological abnormality |
| 1.0–2.9 | 1 | minimal |
| 3.0–6.9 | 2 | mild |
| 7.0–10.9 | 3 | moderate |
| 11.0–13.9 | 4 | marked |
| 14.0–15.0 | 5 | severe |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrini, G.; Siwowska, K.; Haller, S.; Antoine, D.J.; Schibli, R.; Kipar, A.; Müller, C. A Short-Term Biological Indicator for Long-Term Kidney Damage after Radionuclide Therapy in Mice. Pharmaceuticals 2017, 10, 57. https://doi.org/10.3390/ph10020057
Pellegrini G, Siwowska K, Haller S, Antoine DJ, Schibli R, Kipar A, Müller C. A Short-Term Biological Indicator for Long-Term Kidney Damage after Radionuclide Therapy in Mice. Pharmaceuticals. 2017; 10(2):57. https://doi.org/10.3390/ph10020057
Chicago/Turabian StylePellegrini, Giovanni, Klaudia Siwowska, Stephanie Haller, Daniel J. Antoine, Roger Schibli, Anja Kipar, and Cristina Müller. 2017. "A Short-Term Biological Indicator for Long-Term Kidney Damage after Radionuclide Therapy in Mice" Pharmaceuticals 10, no. 2: 57. https://doi.org/10.3390/ph10020057






