Precipitation and Neutralization of Heparin from Different Sources by Protamine Sulfate
Abstract
:1. Introduction
2. Results
2.1. Anticoagulant Activity of Heparin Samples
2.2. Protamine Sulfate Precipitation Assay
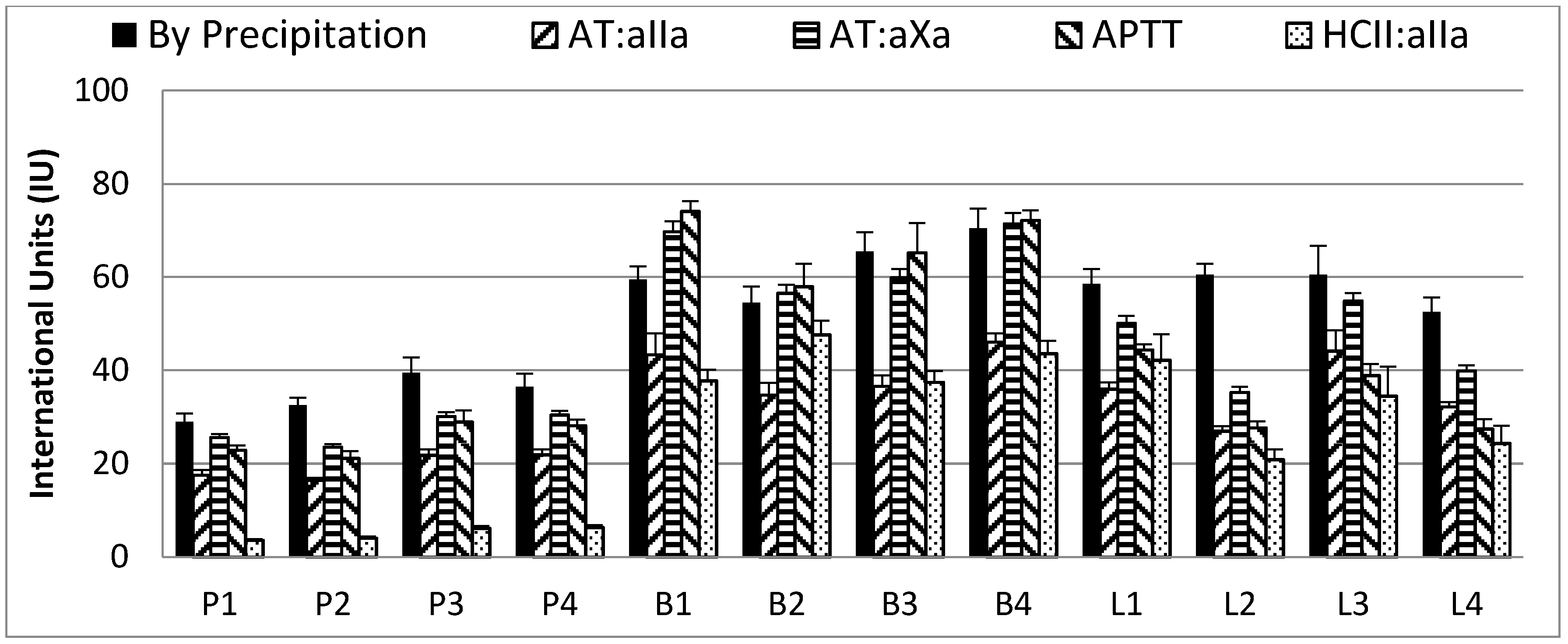
2.3. Neutralization of Heparin Activity
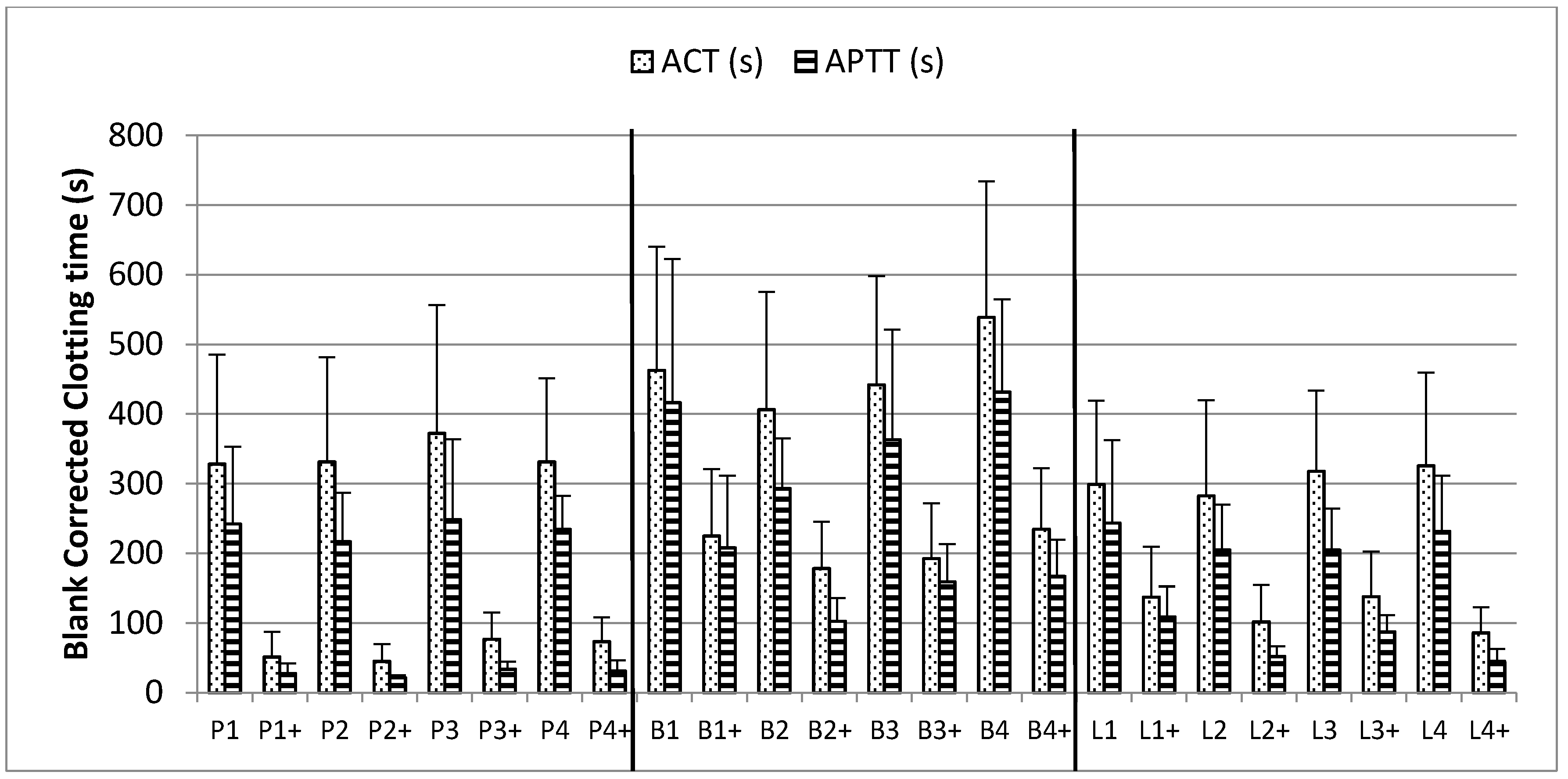
2.4. Thromboelastography
2.5. Molecular Weight
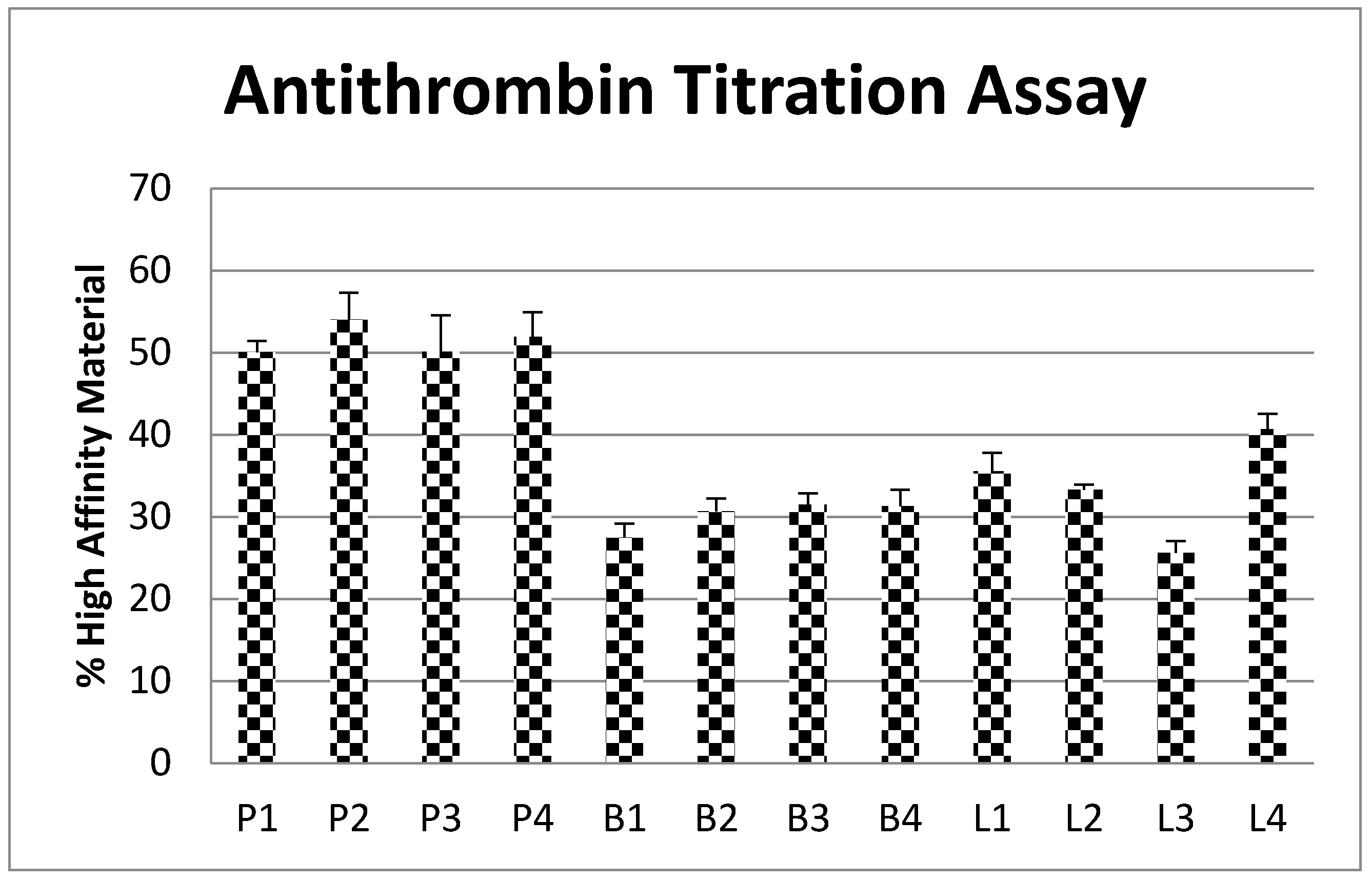
2.6. Antithrombin Titration Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Anticoagulant Assays
4.3. Protamine Sulfate Precipitation Assay
4.4. Heparin/Protamine Sulfate Complex
4.5. Whole Blood Assays
4.6. Molecular Weights
4.7. Antithrombin Fluorescent Titration
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kishimoto, T.K.; Viswanathan, K.; Ganguly, T.; Elankumaran, S.; Smith, S.; Pelzer, K.; Lansing, J.C.; Sriranganathan, N.; Zhao, G.; Galcheva-Gargova, Z.; et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N. Engl. J. Med. 2008, 358, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Szajek, A.Y.; Chess, E.; Johansen, K.; Gratzl, G.; Gray, E.; Keire, D.; Linhardt, R.J.; Liu, J.; Morris, T.; Mulloy, B.; et al. The us regulatory and pharmacopeia response to the global heparin contamination crisis. Nat. Biotechnol. 2016, 34, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Keire, D.A.; Mulloy, B.; Chase, C.; Al-Hakim, A.; Cairatti, D.; Gray, E.; Hogwood, J.; Morris, T.; Mourao, P.; Soares, M.; et al. Diversifying the global heparin supply chain reintroduction of bovine heparin in the united states? Pharm. Technol. 2015, 39, 2–8. [Google Scholar]
- Lindahl, U.; Backstrom, G.; Thunberg, L. The antithrombin-binding sequence in heparin. Identification of an essential 6-O-sulfate group. J. Biol. Chem. 1983, 258, 9826–9830. [Google Scholar] [PubMed]
- Gresele, P.; Busti, C.; Paganelli, G. Heparin in the prophylaxis and treatment of venous thromboembolism and other thrombotic diseases. Handb. Exp. Pharmacol. 2012, 207, 179–209. [Google Scholar]
- Pai, M.; Crowther, M.A. Neutralization of heparin activity. Handb. Exp. Pharmacol. 2012, 207, 265–277. [Google Scholar]
- Chang, L.C.; Lee, H.F.; Yang, Z.; Yang, V.C. Low molecular weight protamine (LMWP) as nontoxic heparin/low molecular weight heparin antidote (i): Preparation and characterization. AAPS PharmSci. 2001, 3, E17. [Google Scholar] [CrossRef] [PubMed]
- Nybo, M.; Madsen, J.S. Serious anaphylactic reactions due to protamine sulfate: A systematic literature review. Basic Clin. Pharmacol. Toxicol. 2008, 103, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Mecca, T.; Consoli, G.M.; Geraci, C.; La Spina, R.; Cunsolo, F. Polycationic calix[8]arenes able to recognize and neutralize heparin. Org. Biomol. Chem. 2006, 4, 3763–3768. [Google Scholar] [CrossRef] [PubMed]
- Kalaska, B.; Kaminski, K.; Sokolowska, E.; Czaplicki, D.; Kujdowicz, M.; Stalinska, K.; Bereta, J.; Szczubialka, K.; Pawlak, D.; Nowakowska, M.; et al. Nonclinical evaluation of novel cationically modified polysaccharide antidotes for unfractionated heparin. PLoS ONE 2015, 10, e0119486. [Google Scholar] [CrossRef] [PubMed]
- Shenoi, R.A.; Kalathottukaren, M.T.; Travers, R.J.; Lai, B.F.; Creagh, A.L.; Lange, D.; Yu, K.; Weinhart, M.; Chew, B.H.; Du, C.; et al. Affinity-based design of a synthetic universal reversal agent for heparin anticoagulants. Sci. Transl. Med. 2014, 6, 260ra150. [Google Scholar] [CrossRef] [PubMed]
- Valimaki, S.; Khakalo, A.; Ora, A.; Johansson, L.S.; Rojas, O.J.; Kostiainen, M.A. Effect of peg-pdmaema block copolymer architecture on polyelectrolyte complex formation with heparin. Biomacromolecules 2016, 17, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B.; Gray, E.; Barrowcliffe, T.W. Characterization of unfractionated heparin: Comparison of materials from the last 50 years. Thromb. Haemost. 2000, 84, 1052–1056. [Google Scholar] [PubMed]
- St Ange, K.; Onishi, A.; Fu, L.; Sun, X.; Lin, L.; Mori, D.; Zhang, F.; Dordick, J.S.; Fareed, J.; Hoppensteadt, D.; et al. Analysis of heparins derived from bovine tissues and comparison to porcine intestinal heparins. Clin. Appl. Thromb. Hemost. 2016, 22, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Bertini, S.; Fareed, J.; Madaschi, L.; Risi, G.; Torri, G.; Naggi, A. Characterization of pf4-heparin complexes by photon correlation spectroscopy and zeta potential. Clin. Appl. Thromb. Hemost. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tovar, A.M.; Capille, N.V.; Santos, G.R.; Vairo, B.C.; Oliveira, S.N.; Fonseca, R.J.; Mourao, P.A. Heparin from bovine intestinal mucosa: Glycans with multiple sulfation patterns and anticoagulant effects. Thromb. Haemost. 2012, 107, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.D.; Ye, H.; Liu, J.; Linhardt, R.J.; Keire, D.A. Heparin and homogeneous model heparin oligosaccharides form distinct complexes with protamine: Light scattering and zeta potential analysis. J. Pharm. Biomed. Anal. 2017, 140, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, N.; Baliga, N.; Wakefield, T.W.; Andrews, P.C.; Yang, V.C.; Meyerhoff, M.E. Determination of low-molecular-weight heparins and their binding to protamine and a protamine analog using polyion-sensitive membrane electrodes. Anal. Biochem. 1999, 266, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.; Hogwood, J.; Gray, E.; Mulloy, B.; Hackett, A.M.; Johansen, K.B. Protamine neutralisation of low molecular weight heparins and their oligosaccharide components. Anal. Bioanal. Chem. 2011, 399, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Tovar, A.M.; Santos, G.R.; Capille, N.V.; Piquet, A.A.; Glauser, B.F.; Pereira, M.S.; Vilanova, E.; Mourao, P.A. Structural and haemostatic features of pharmaceutical heparins from different animal sources: Challenges to define thresholds separating distinct drugs. Sci. Rep. 2016, 6, 35619. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, P.; Rayamajhi, S.; Verma, V.; Gundabolu, K.; Bhatt, V.R. Reversal of anticoagulation and management of bleeding in patients on anticoagulants. Clin. Appl. Thromb. Hemost. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Hogwood, J.; Mulloy, B. The anticoagulant and antithrombotic mechanisms of heparin. Handb. Exp. Pharmacol. 2012, 207, 43–61. [Google Scholar]
- Panagos, C.G.; Thomson, D.S.; Moss, C.; Hughes, A.D.; Kelly, M.S.; Liu, Y.; Chai, W.; Venkatasamy, R.; Spina, D.; Page, C.P.; et al. Fucosylated chondroitin sulfates from the body wall of the sea cucumber holothuria forskali: Conformation, selectin binding, and biological activity. J. Biol. Chem. 2014, 289, 28284–28298. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B.; Hogwood, J. Chromatographic molecular weight measurements for heparin, its fragments and fractions, and other glycosaminoglycans. Methods Mol. Biol. 2015, 1229, 105–118. [Google Scholar] [PubMed]
- Boothello, R.S.; Al-Horani, R.A.; Desai, U.R. Glycosaminoglycan-protein interaction studies using fluorescence spectroscopy. Methods Mol. Biol. 2015, 1229, 335–353. [Google Scholar] [PubMed]
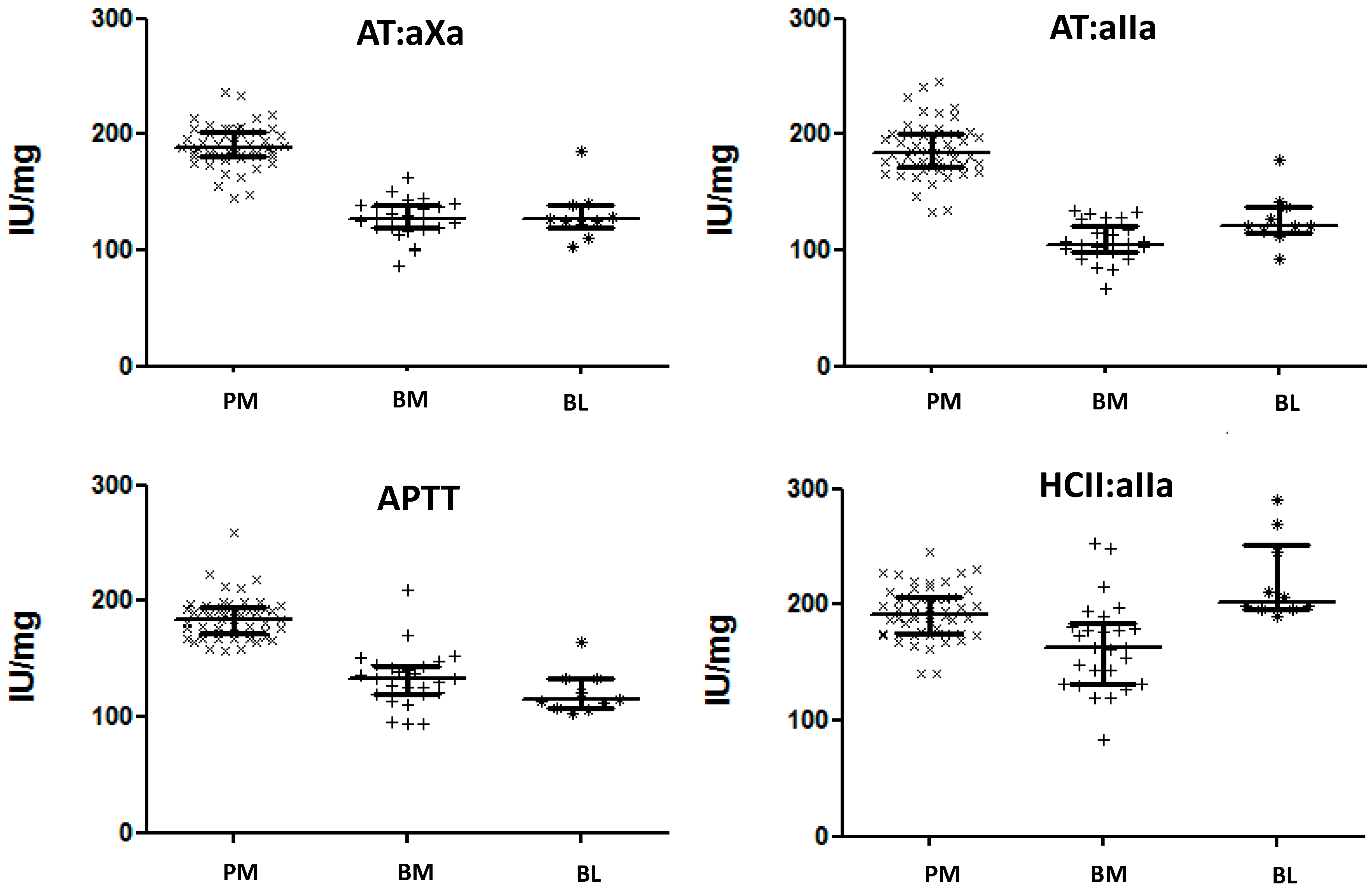
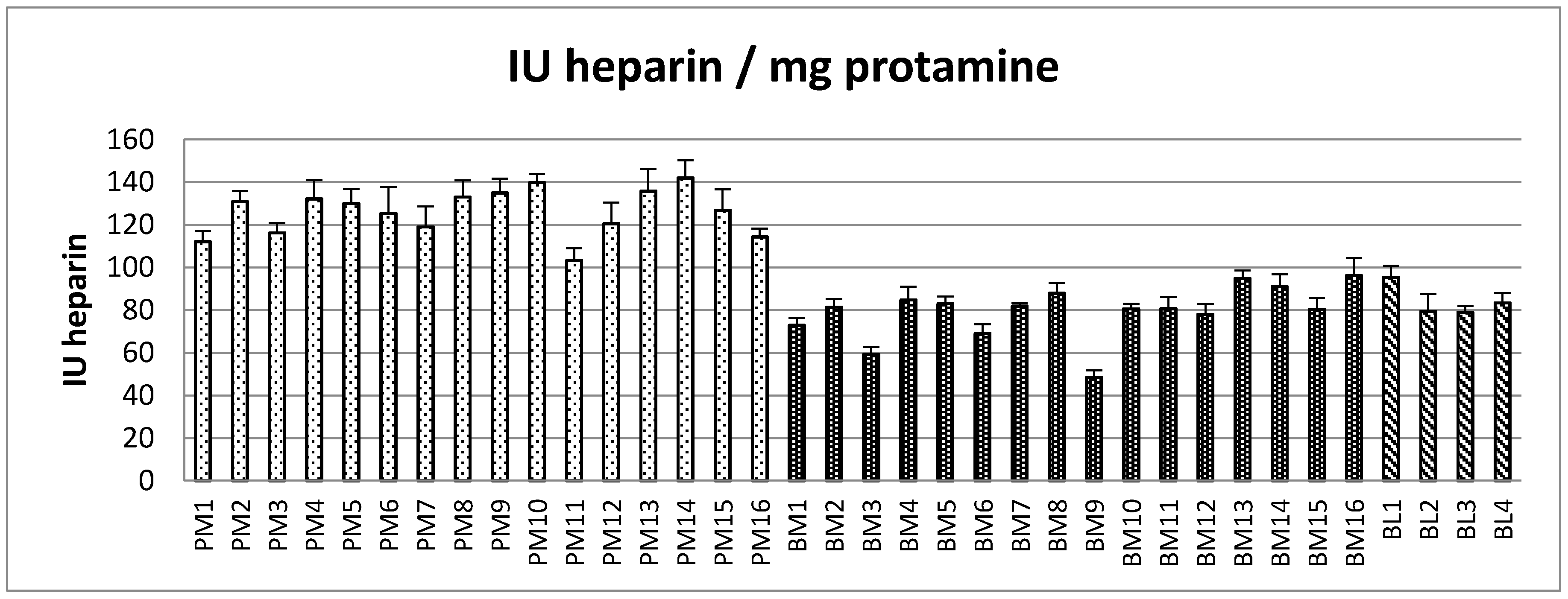
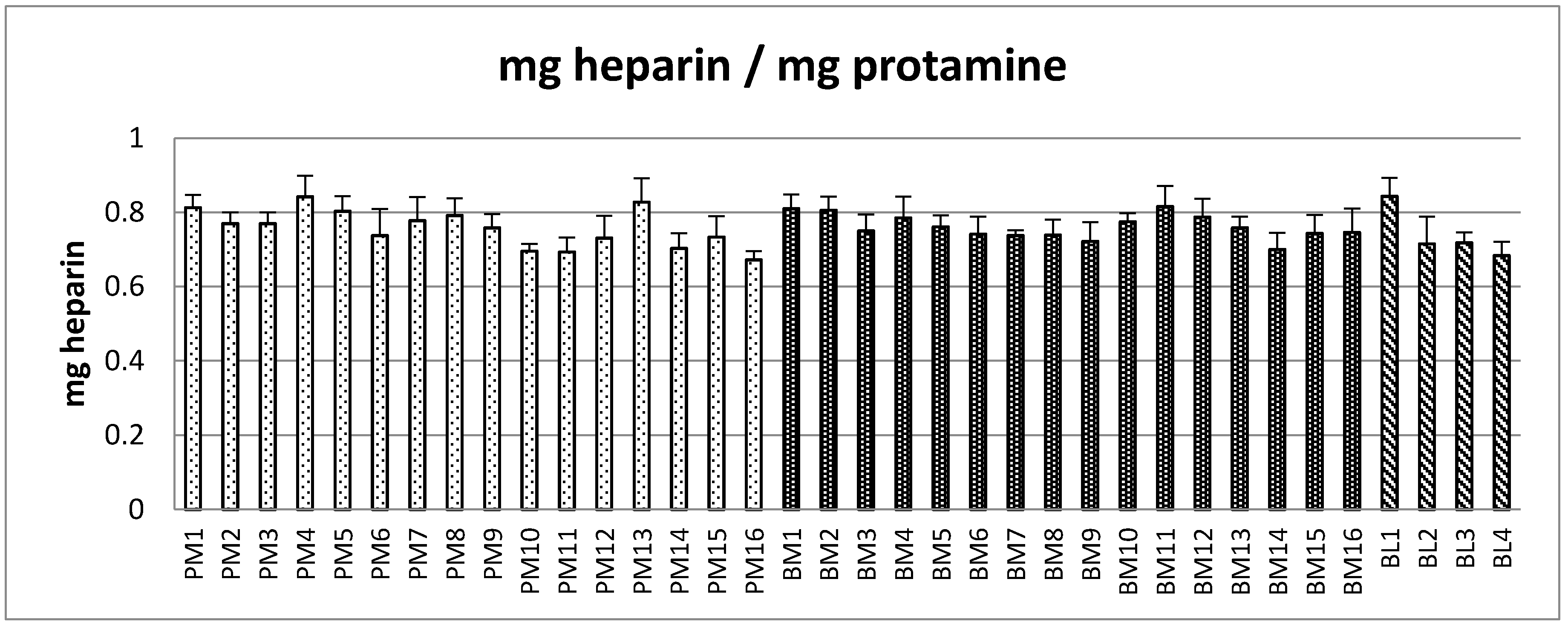
| PM (n = 16) | BM (n = 16) | BL (n = 4) | p-Values | |
|---|---|---|---|---|
| IU/mg PS Mean (range) | 126 (103–142) | 79.4 (48.4–96.2) | 84.3 (79.0–95.3) | PM–BM < 0.001; PM–BL < 0.001; BM–BL = 0.233 |
| mg/mg PS Mean (range) | 0.76 (0.67–0.84) | 0.76 (0.70–0.82) | 0.74 (0.68–0.84) | PM–BM = 0.403; PM–BL = 0.289; BM–BL = 0.188 |
| Sample Code and IU Heparin/mg Protamine | ||||
|---|---|---|---|---|
| PM | P1 | P2 | P3 | P4 |
| 142 | 135 | 121 | 127 | |
| BM | B1 | B2 | B3 | B4 |
| 81 | 91 | 69 | 59 | |
| BL | L1 | L2 | L3 | L4 |
| 81 | 79 | 79 | 95 | |
| By EP Precipitation Assay | By Neutralization of Biological Activity | |||
|---|---|---|---|---|
| Ave (n = 4) | IU anti-IIa/mg protamine | mg heparin/mg protamine | IU anti-IIa/mg protamine | mg heparin/mg protamine |
| PM | 131 (±9) | 0.73 (±0.02) | 161 (±6) | 0.90 (±0.06) |
| BM | 75 (±14) | 0.75 (±0.04) | 123 (±9) | 1.26 (±0.19) |
| BL | 84 (±8) | 0.74 (±0.07) | 131 (±15) | 1.16 (±0.15) |
| Mp | Mn | Mw | PD | % < 8 kDa | ||
|---|---|---|---|---|---|---|
| Porcine Mucosa | P1 | 15,800 | 13,820 | 17,870 | 1.29 | 8.7 |
| P2 | 16,110 | 13,900 | 17,290 | 1.24 | 7.7 | |
| P3 | 16,320 | 14,140 | 17,390 | 1.23 | 6.5 | |
| P4 | 15,080 | 13,080 | 15,720 | 1.2 | 8.4 | |
| Bovine Mucosa | B1 | 13,230 | 11,660 | 14,750 | 1.27 | 15.5 |
| B2 | 16,140 | 14,980 | 17,950 | 1.2 | 4.4 | |
| B3 | 14,130 | 12,920 | 15,920 | 1.23 | 9.4 | |
| B4 | 13,940 | 12,670 | 15,430 | 1.22 | 9.8 | |
| Bovine Lung | L1 | 9700 | 11,280 | 14,450 | 1.28 | 18.1 |
| L2 | 8770 | 10,570 | 13,380 | 1.27 | 22.7 | |
| L3 | 8810 | 9740 | 12,160 | 1.25 | 28 | |
| L4 | 9720 | 11,310 | 14,360 | 1.27 | 17.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hogwood, J.; Mulloy, B.; Gray, E. Precipitation and Neutralization of Heparin from Different Sources by Protamine Sulfate. Pharmaceuticals 2017, 10, 59. https://doi.org/10.3390/ph10030059
Hogwood J, Mulloy B, Gray E. Precipitation and Neutralization of Heparin from Different Sources by Protamine Sulfate. Pharmaceuticals. 2017; 10(3):59. https://doi.org/10.3390/ph10030059
Chicago/Turabian StyleHogwood, John, Barbara Mulloy, and Elaine Gray. 2017. "Precipitation and Neutralization of Heparin from Different Sources by Protamine Sulfate" Pharmaceuticals 10, no. 3: 59. https://doi.org/10.3390/ph10030059





