Comparison Study of Two Differently Clicked 18F-Folates—Lipophilicity Plays a Key Role
Abstract
:1. Introduction
2. Results
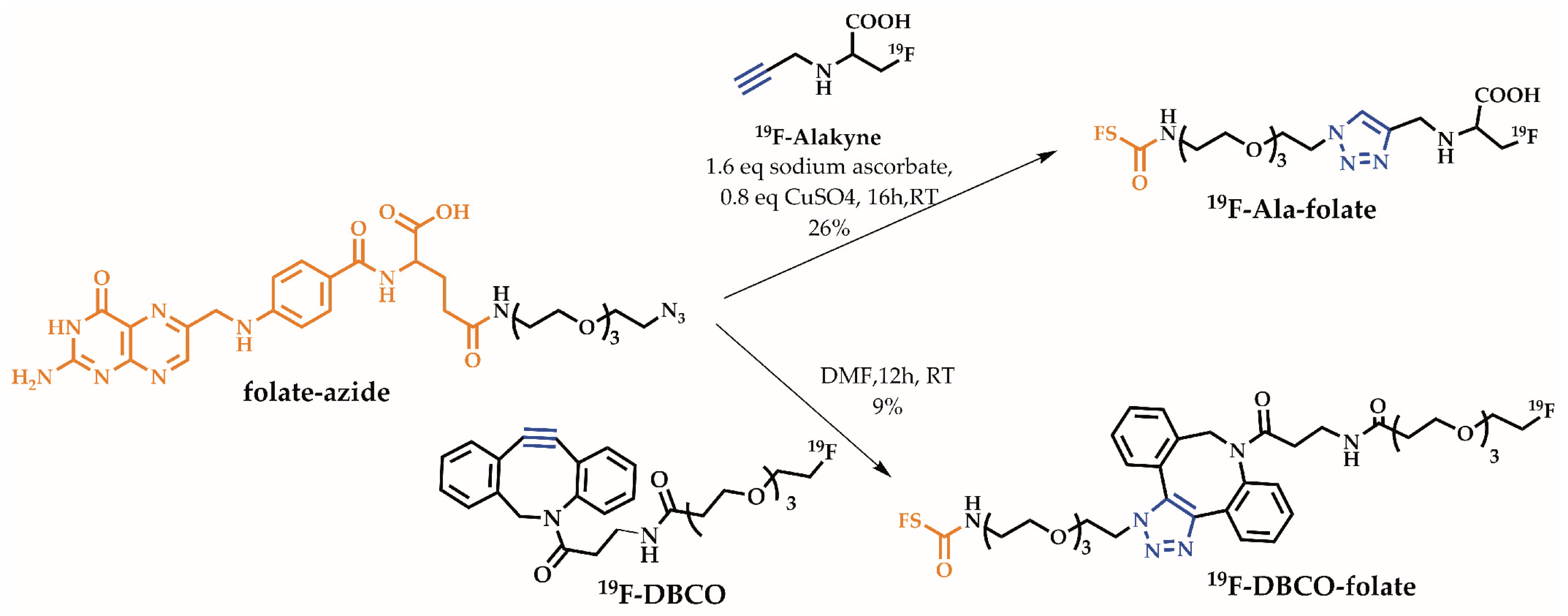
2.1. Organic Chemistry
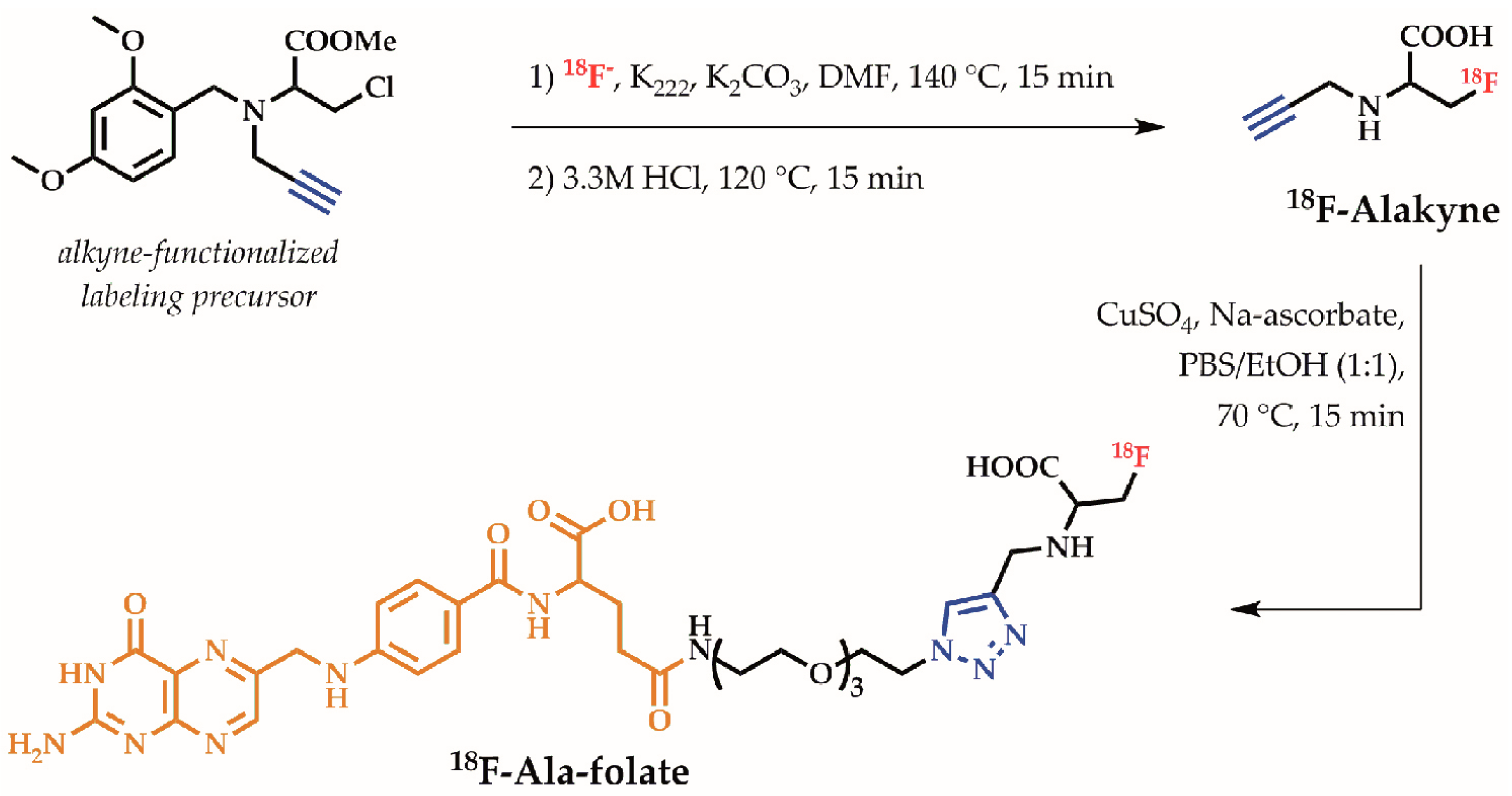
2.2. Radiochemistry
2.3. Lipophilicity
3. In Vitro Studies
3.1. Stability Studies in Human Serum Albumin
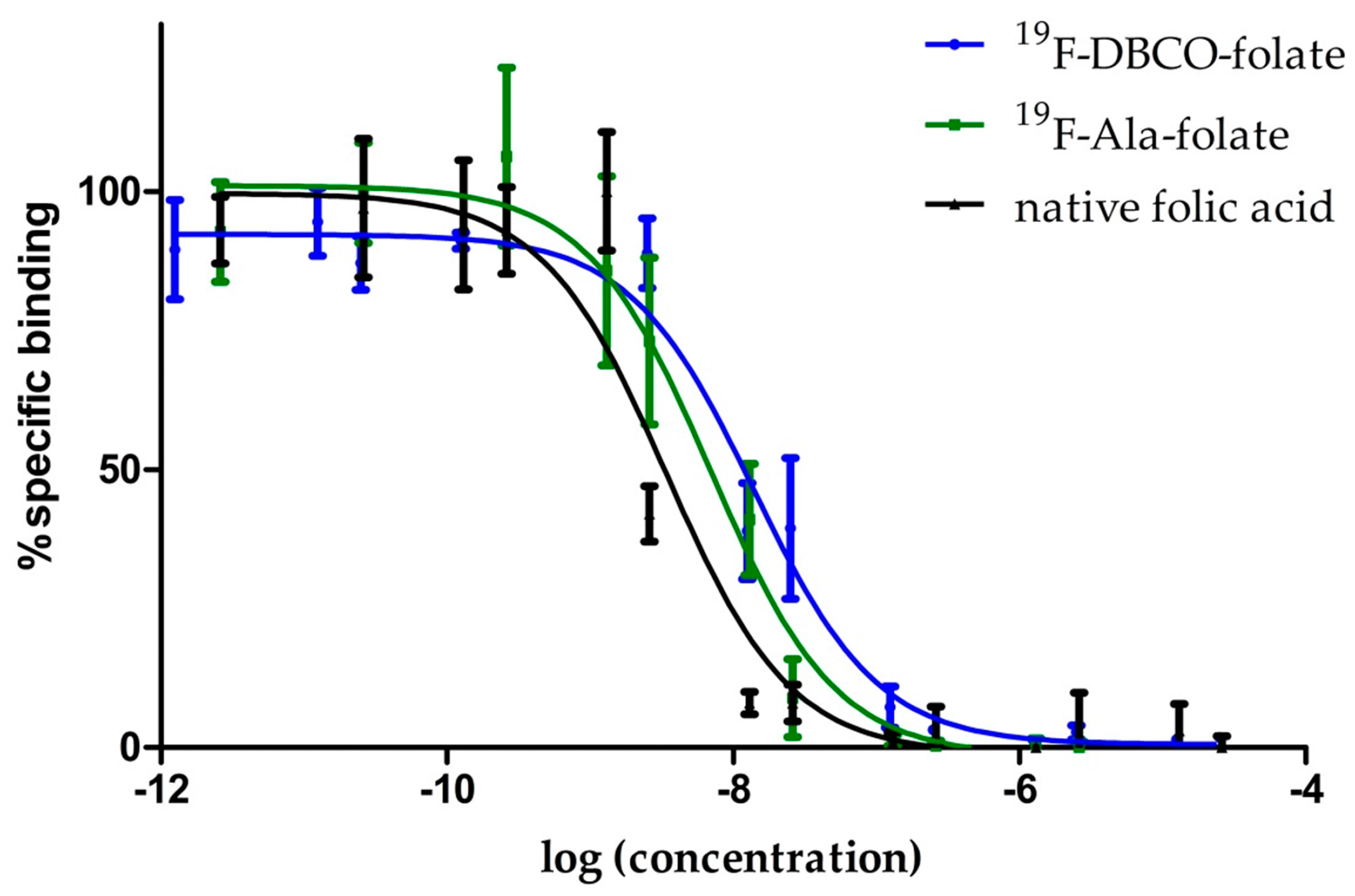
3.2. Binding Affinity Studies
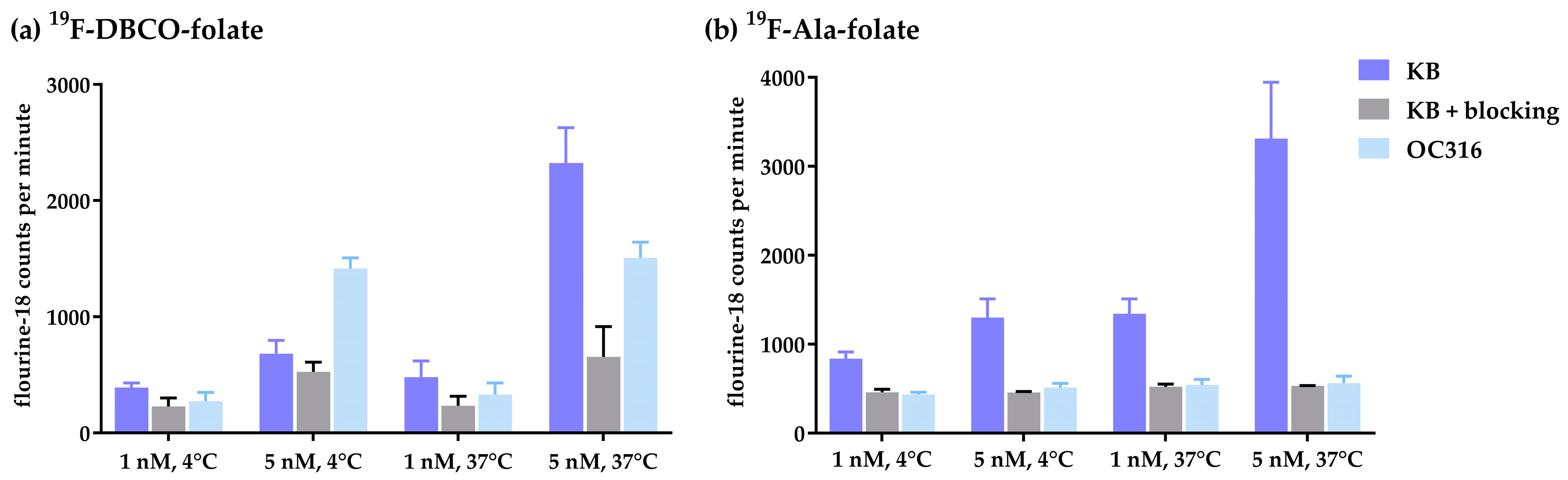
3.3. Internalization Studies
4. Animal Studies
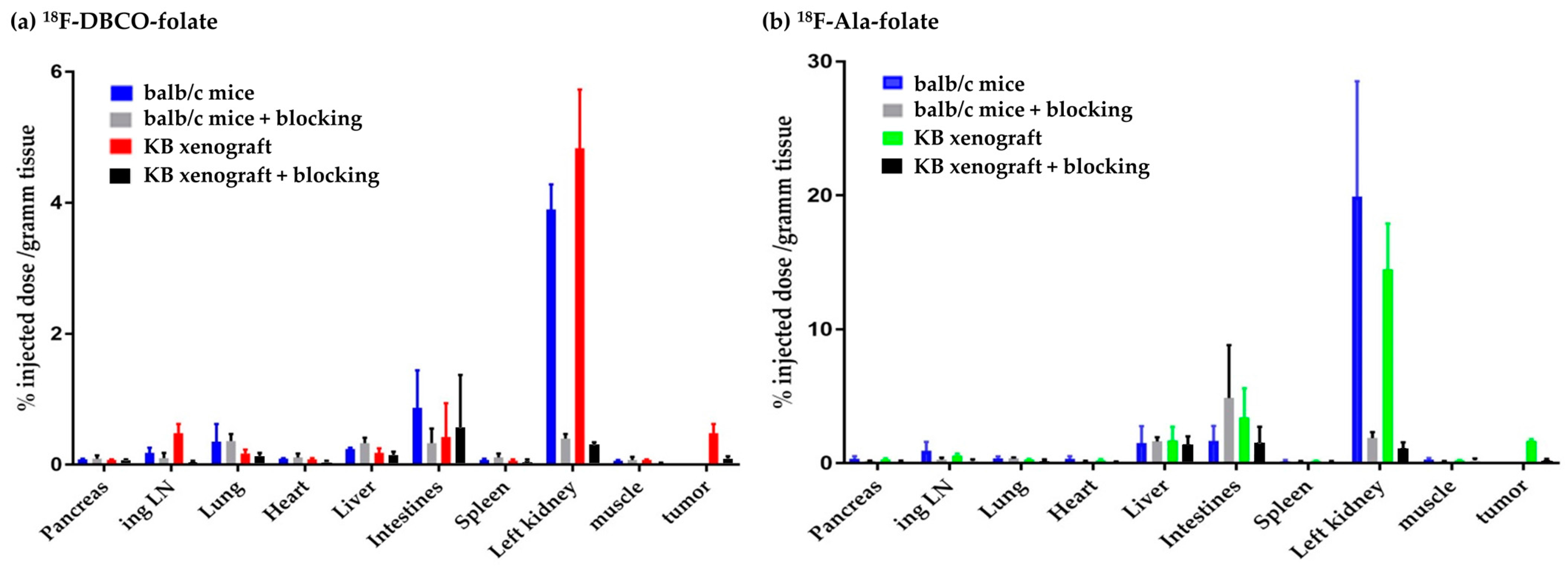
4.1. Ex Vivo Biodistribution Studies
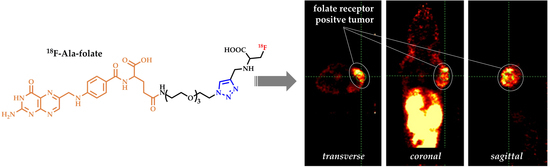
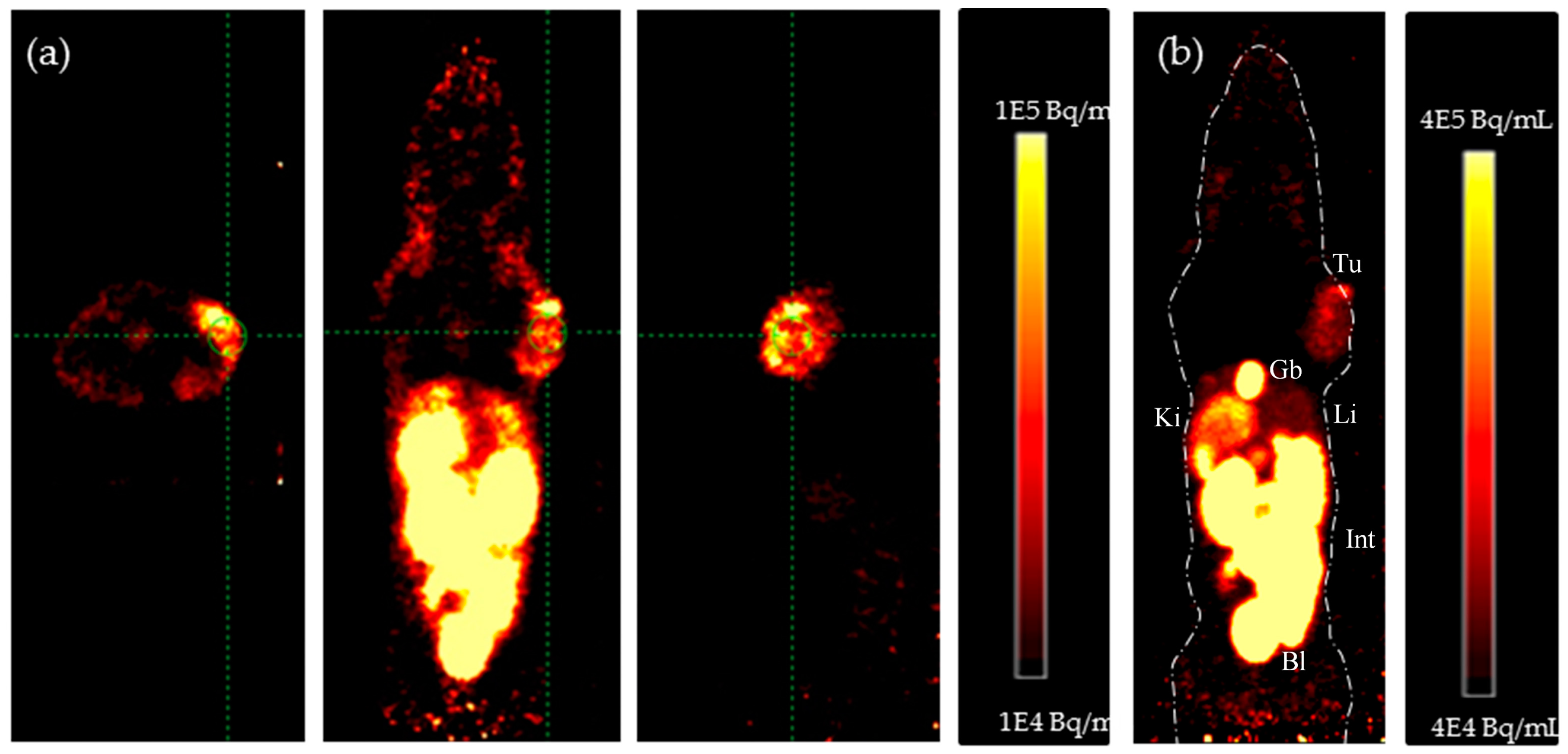
4.2. In Vivo PET Studies
5. Discussion
6. Materials and Methods
6.1. Materials
6.2. Organic Chemistry
6.3. Radiochemistry: Labeling with n.c.a. [18F]Fluoride
6.3.1. 18F-Ala-Folate
6.3.2. 18F-DBCO-Folate
6.4. Determination of Lipophilicty
6.5. In Vitro Studies
6.5.1. Stability Studies in Human Serum Albumin
6.6. In Vivo Studies
7. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schirch, V.; Strong, W.B. Interaction of folylpolyglutamates with enzymes in one-carbon metabolism. Arch. Biochem. Biophys. 1989, 269, 371–380. [Google Scholar] [CrossRef]
- Antony, A.C. Folate Receptors. Annu. Rev. Nutr. 1996, 16, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.; Inoue, K.; Hayashi, Y. Molecular and functional characteristics of proton-coupled folate transporter. J. Pharm. Sci. 2009, 98, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Whetstine, J.R.; Flatley, R.M.; Matherly, L.H. The human reduced folate carrier gene is ubiquitously and differentially expressed in normal human tissues: Identification of seven non-coding exons and characterization of a novel promoter. Biochem. J. 2002, 367, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.Y.; Mathias, C.J.; Green, M.A. The folate receptor as a molecular target for tumor-selective radionuclide delivery. Nucl. Med. Biol. 2003, 30, 811–817. [Google Scholar] [CrossRef]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R., Jr.; Kamen, B. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues distribution of the folate receptor GP38 in normal and malignant cell Lines and tissue. Cancer Res. 1992, 52, 3396–3401. [Google Scholar] [PubMed]
- Weitman, S.D.; Weinberg, A.G.; Coney, L.R.; Zurawski, V.R.; Jennings, D.S.; Kamen, B.A. Cellular localization of the folate receptor: Potential role in drug toxicity and folate homeostasis. Cancer Res. 1992, 52, 6708–6711. [Google Scholar] [PubMed]
- Ross, T.L.; Honer, M.; Lam, P.Y.H.; Mindt, T.L.; Groehn, V.; Schibli, R.; Schubiger, P.A.; Ametamey, S.M. Fluorine-18 click radiosynthesis and preclinical evaluation of a new 18F-labeled folic acid derivative. Bioconjug. Chem. 2008, 19, 2462–2470. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.R.; Müller, C.; Rebert, J.; Müller, A.; Kramer, S.D.; Ametamey, S.M.; Schibli, R. [18F]fluoro-deoxy-glucose folate: a novel PET radiotracer with improved in vivo properties for folate receptor targeting. Bioconjug. Chem. 2012, 23, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Jammaz, I. Al.; Al-Otaibi, B.; Amer, S.; Al-Hokbany, N.; Okarvi, S. Novel synthesis and preclinical evaluation of folic acid derivatives labeled with 18F-FDG for PET imaging of folate receptor-positive tumors. Nucl. Med. Biol. 2012, 39, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Schieferstein, H.; Betzel, T.; Fischer, C.R.; Ross, T.L. F-click labeling and preclinical evaluation of a new 18 F-folate for PET imaging. EJNMMI 2013, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bettio, A.; Honer, M.; Müller, C.; Bruhlmeier, M.; Müller, U.; Schibli, R.; Groehn, V.; Schubiger, A.P.; Ametamey, S.M. Synthesis and preclinical evaluation of a folic acid derivative labeled with 18F for PET imaging of folate receptor-positive tumors. J. Nucl. Med. 2006, 47, 1153–1160. [Google Scholar] [PubMed]
- Betzel, T.; Müller, C.; Groehn, V.; Müller, A.; Rebert, J.; Fischer, C.R.; Kramert, S.D.; Schibli, R.; Ametamet, S.M. Radiosynthesis and Preclinical Evaluation of 3′-Aza-2′-[18F]fluorofolic Acid: A Novel PET Radiotracer for Folate Receptor Targeting. Bioconjug. Chem. 2013, 24, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.L.; Honer, M.; Mu, C.; Groehn, V.; Schibli, R.; Ametamey, S.M. A new 18F-labeled folic acid derivative with improved properties for the PET imaging of folate receptor-positive tumors. J. Nucl. Med. 2010, 51, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.R.; Groehn, V.; Reber, J.; Schibli, R.; Ametamey, S.M.; Müller, C. Improved PET imaging of tumors in mice using a novel 18F-folate conjugate with an albumin-binding entity. Mol. Imaging Biol. 2013, 15, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Schieferstein, H.; Ross, T.L. A polar 18F-labeled amino acid derivative for click labeling of biomolecules. Eur. J. Org. Chem. 2014, 17, 3546–3550. [Google Scholar] [CrossRef]
- Kettenbach, K.; Ross, T.L. A 18F-labeled dibenzocyclooctyne (DBCO) derivative for copper-free click labeling of biomolecules. Med. Chem. Commun. 2016, 7, 654–657. [Google Scholar] [CrossRef]
- Temple, C.; Rose, J.D.; Montgomery, J.A. Chemical conversion of folic acid to pteroic acid. J. Org. Chem. 1981, 508, 3666–3667. [Google Scholar] [CrossRef]
- Kettenbach, K.; Schieferstein, H.; Grunewald, C.; Iffland, D.; Reffert, L.M.; Hampel, G.; Schütz, C.L.; Bings, N.H.; Ross, T.L. Synthesis and evaluation of boron folates for Boron-Neutron-Capture-Therapy (BNCT). Radiochim. Acta 2015, 103, 799–809. [Google Scholar] [CrossRef]
- Waterhouse, R.N. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2003, 5, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Boss, S.D.; Betzel, T.; Müller, C.; Fischer, C.R.; Haller, S.; Reber, J.; Groehn, V.; Schibli, R.; Ametamey, S.M. Comparative studies of three pairs of α- and γ-conjugated folic acid derivatives labeled with fluorine-18. Bioconjug. Chem. 2015, 27, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.S.; Meng, X.J.; McQuade, P.; Rubins, D.; Lin, S.-A.; Zeng, Z.Z.; Haley, H.; Miller, P.; Trotter, D.G.; Low, P.S. Synthesis and Preclinical Evaluation of Folate-NOTA-Al18F for PET Imaging of Folate-Receptor-Positive Tumors. Mol. Pharm. 2016, 13, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Hausner, S.H.; Carpenter, R.D.; Bauer, N.; Sutcliffe, J.L. Evaluation of an integrin αvβ6-specific peptide labeled with [18F]fluorine by copper-free, strain-promoted click chemistry. Nucl. Med. Biol. 2013, 40, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Leamon, C.P.; Reddy, J.A.; Dorton, R.; Bloomfield, A.; Emsweller, K.; Parker, N.; Westrick, E. Impact of high and low folate diets on tissue folate receptor levels and antitumor responses toward folate-drug conjugates. J. Pharmacol. Exp. Ther. 2008, 327, 918–925. [Google Scholar] [CrossRef] [PubMed]

| Substance | logD7.4 Value |
|---|---|
| 18F-DBCO | 1.2 ± 0.07 [18] |
| 18F-DBCO-folate | 0.6 ± 0.07 [18] |
| 18F-Alakyne | −1.18 ± 0.03 |
| 18F-Ala-folate | −1.43 ± 0.08 |
| Substance | k′ Value | Retention Time (min) |
|---|---|---|
| native folic acid | 0.3 [5] | 3.07 |
| 19F-DBCO-folate | 0.50 ± 0.10 | 3.26 |
| 19F-Ala-folate | 0.27 | 2.89 |
| Substance | IC50 [nM] | pIC50 | Ki [nM] 1 |
|---|---|---|---|
| native folic acid | 1.9 | 8.72 | 1.6 nM |
| 19F-DBCO-folate | 11.2 ± 3.7 | 7.98 ± 0.21 | 6.3 ±1.4 |
| 19F-Ala-folate | 6.4 ± 0.5 | 8.20 ± 0.05 | 5.5 ± 0.4 |
| Ratio | 18F-DBCO-Folate | 18F-Ala-Folate |
|---|---|---|
| tumor/blood | 5.61 | 10.11 |
| tumor/liver * | 2.58 | 0.98 |
| tumor/kidney | 0.05 | 0.12 |
| tumor/muscle | 6.86 | 7.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kettenbach, K.; Reffert, L.M.; Schieferstein, H.; Pektor, S.; Eckert, R.; Miederer, M.; Rösch, F.; Ross, T.L. Comparison Study of Two Differently Clicked 18F-Folates—Lipophilicity Plays a Key Role. Pharmaceuticals 2018, 11, 30. https://doi.org/10.3390/ph11010030
Kettenbach K, Reffert LM, Schieferstein H, Pektor S, Eckert R, Miederer M, Rösch F, Ross TL. Comparison Study of Two Differently Clicked 18F-Folates—Lipophilicity Plays a Key Role. Pharmaceuticals. 2018; 11(1):30. https://doi.org/10.3390/ph11010030
Chicago/Turabian StyleKettenbach, Kathrin, Laura M. Reffert, Hanno Schieferstein, Stefanie Pektor, Raphael Eckert, Matthias Miederer, Frank Rösch, and Tobias L. Ross. 2018. "Comparison Study of Two Differently Clicked 18F-Folates—Lipophilicity Plays a Key Role" Pharmaceuticals 11, no. 1: 30. https://doi.org/10.3390/ph11010030