Improved Intranasal Retentivity and Transnasal Absorption Enhancement by PEGylated Poly-l-ornithine
Abstract
:1. Introduction
2. Results
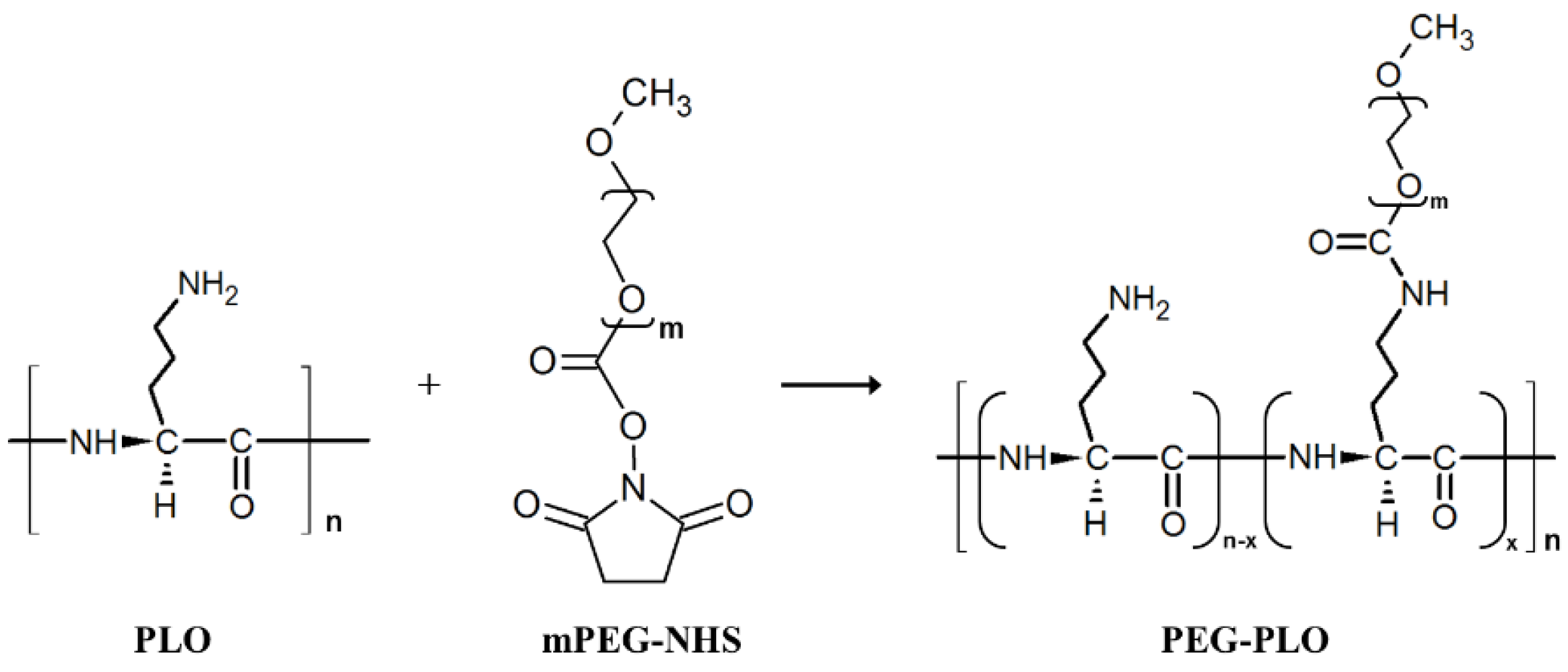
2.1. Synthesis of PEGylated-PLO (PEG-PLO)
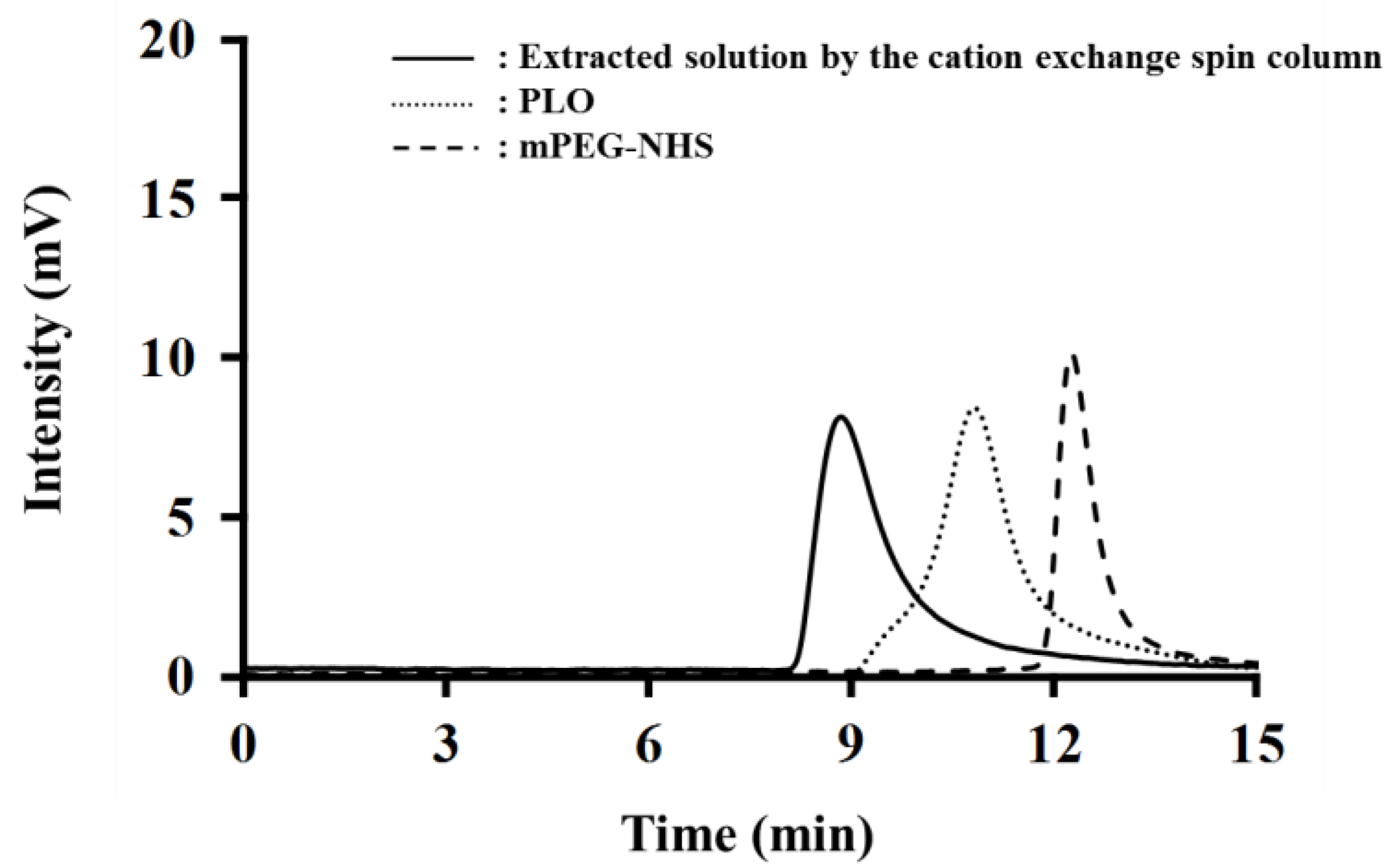
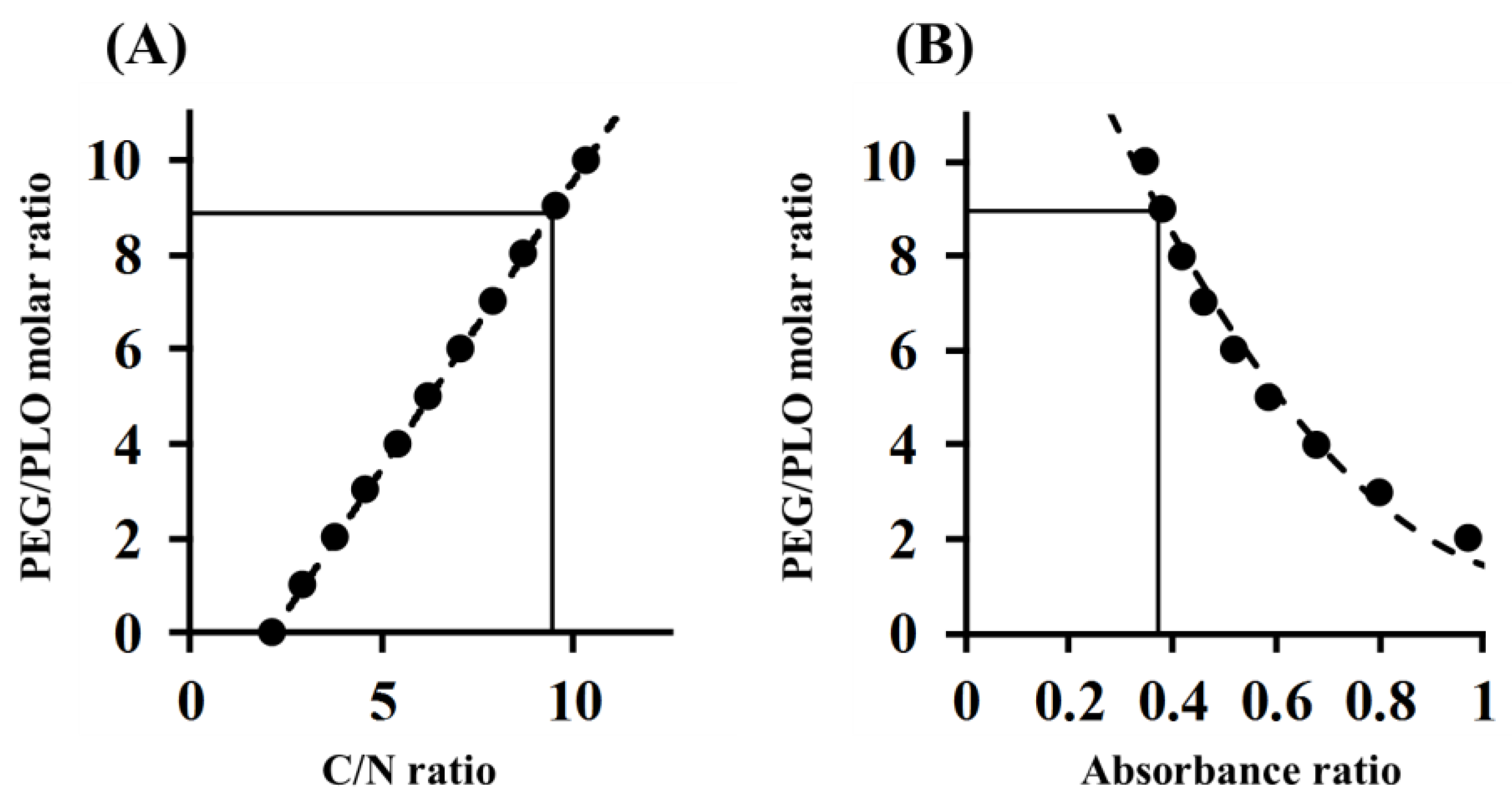
2.2. Determination of the PEGylation Ratio of PEG-PLO
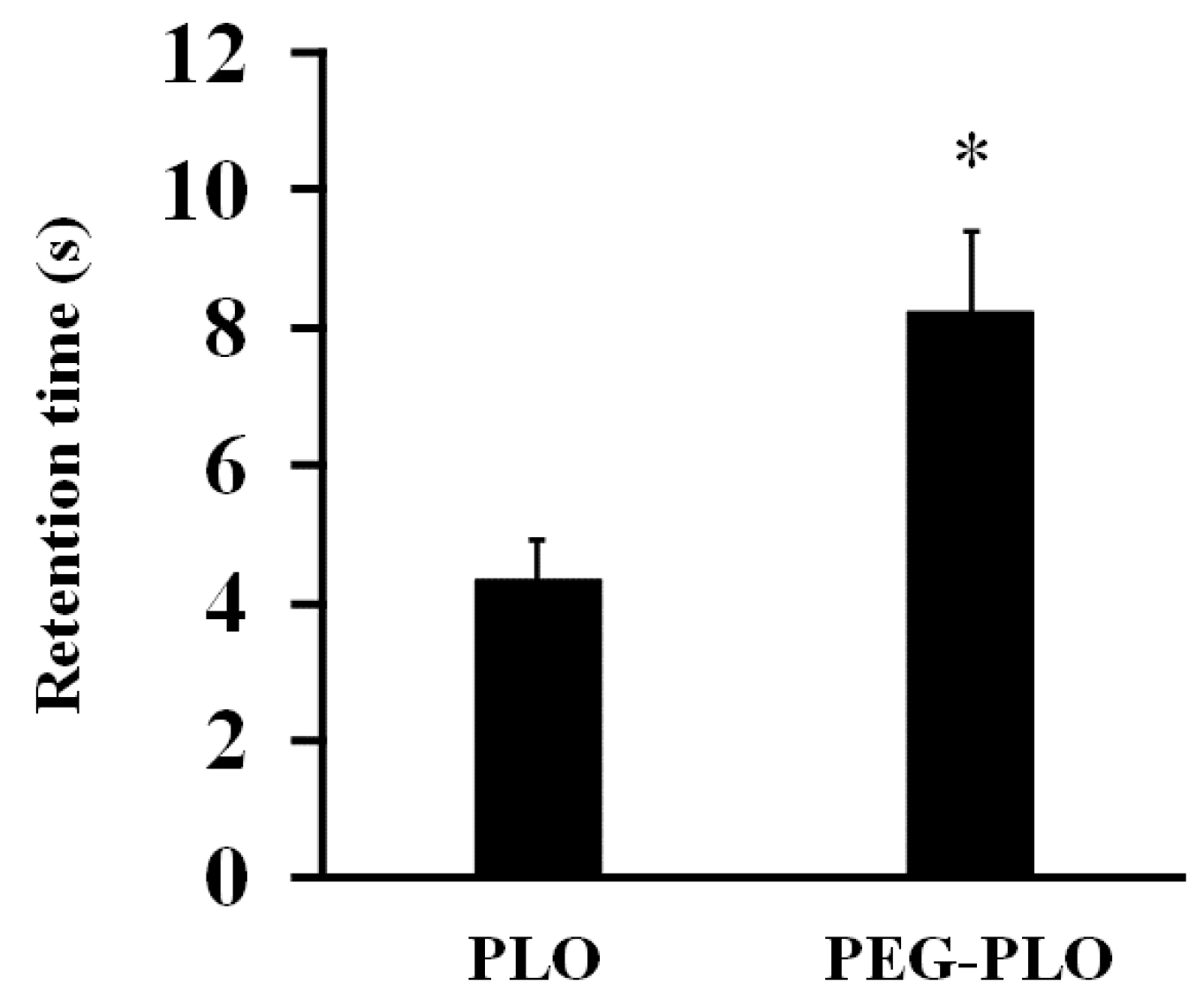
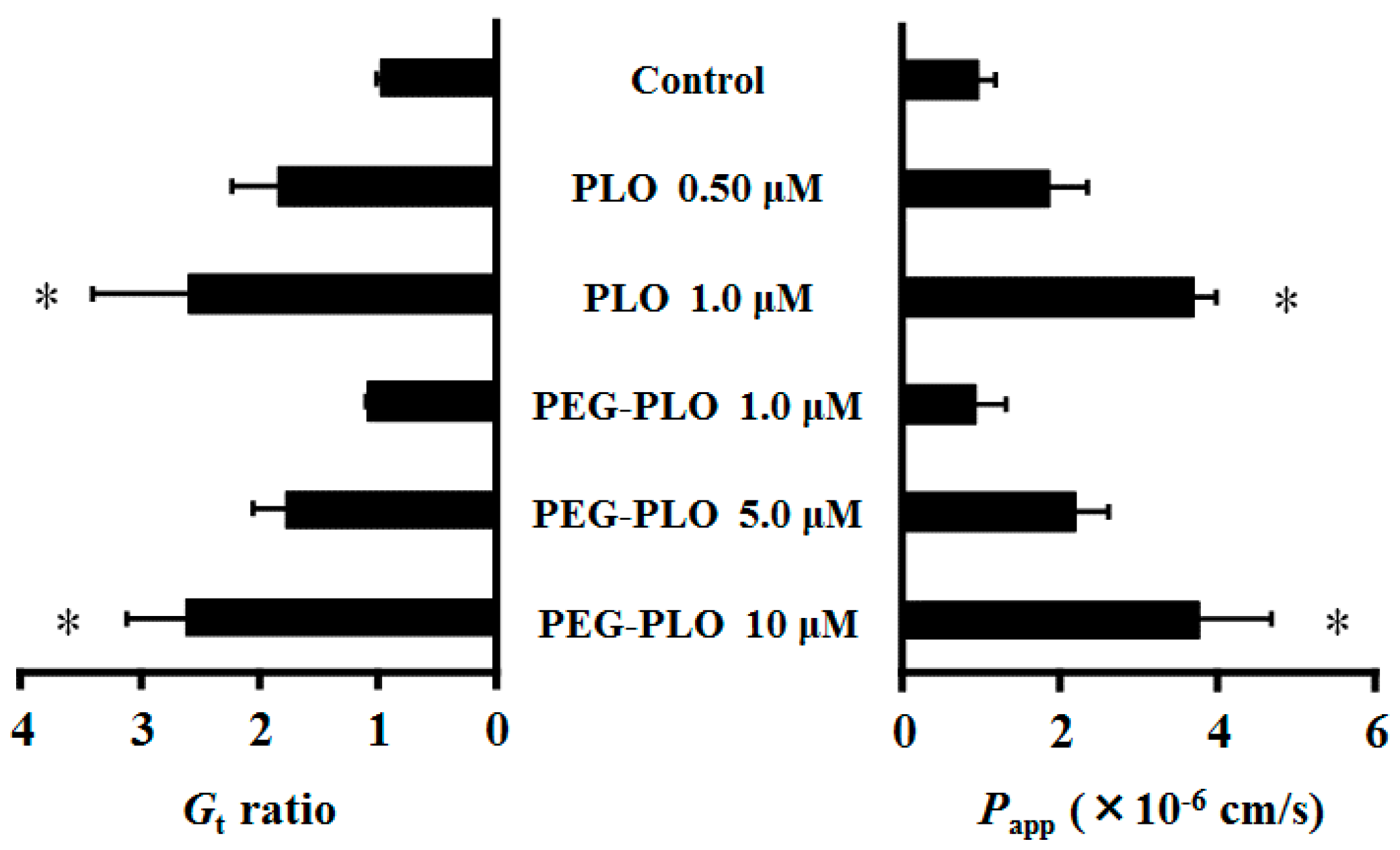
2.3. Impact of PEGylation of PLO on In Vitro Retentivity
2.4. Effects of PEG-PLO on Fluorescein Isothiocyanate Dextran (FD-4) Permeation Across Caco-2 Cells
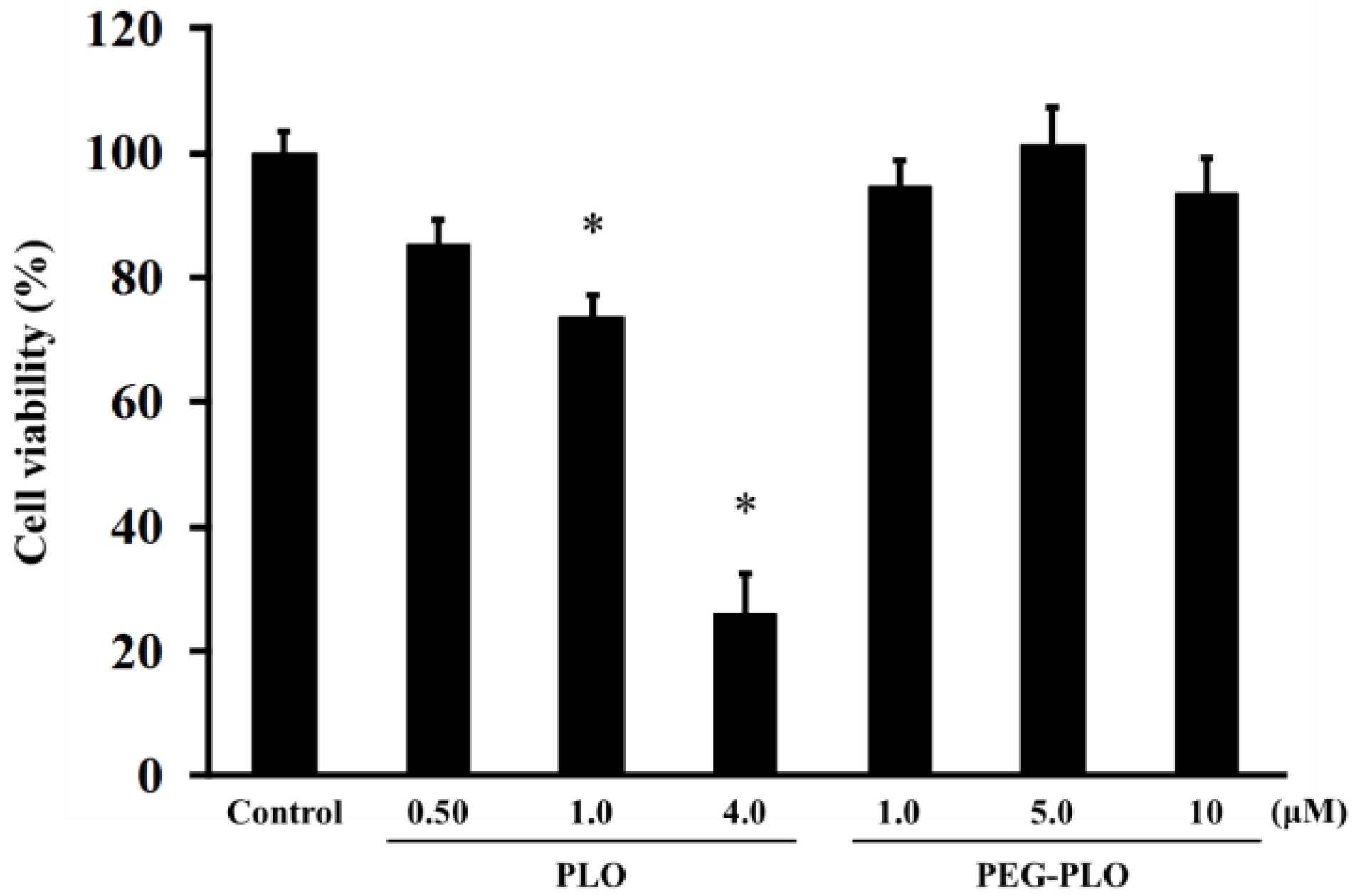
2.5. Cell Viability after Application of PEG-PLO
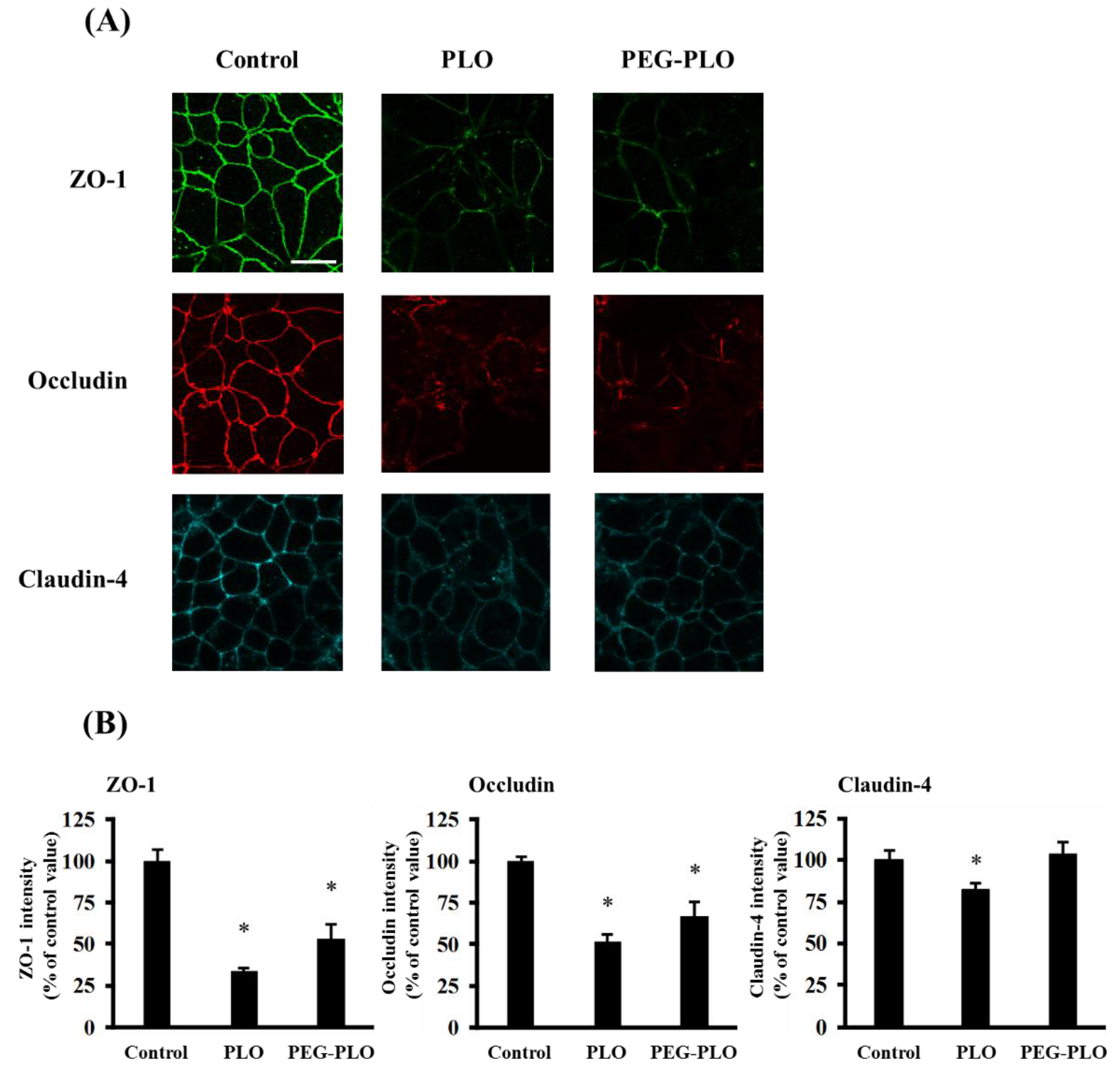
2.6. Tight Junction (TJ) Protein Localization after Application of PLO and PEG-PLO
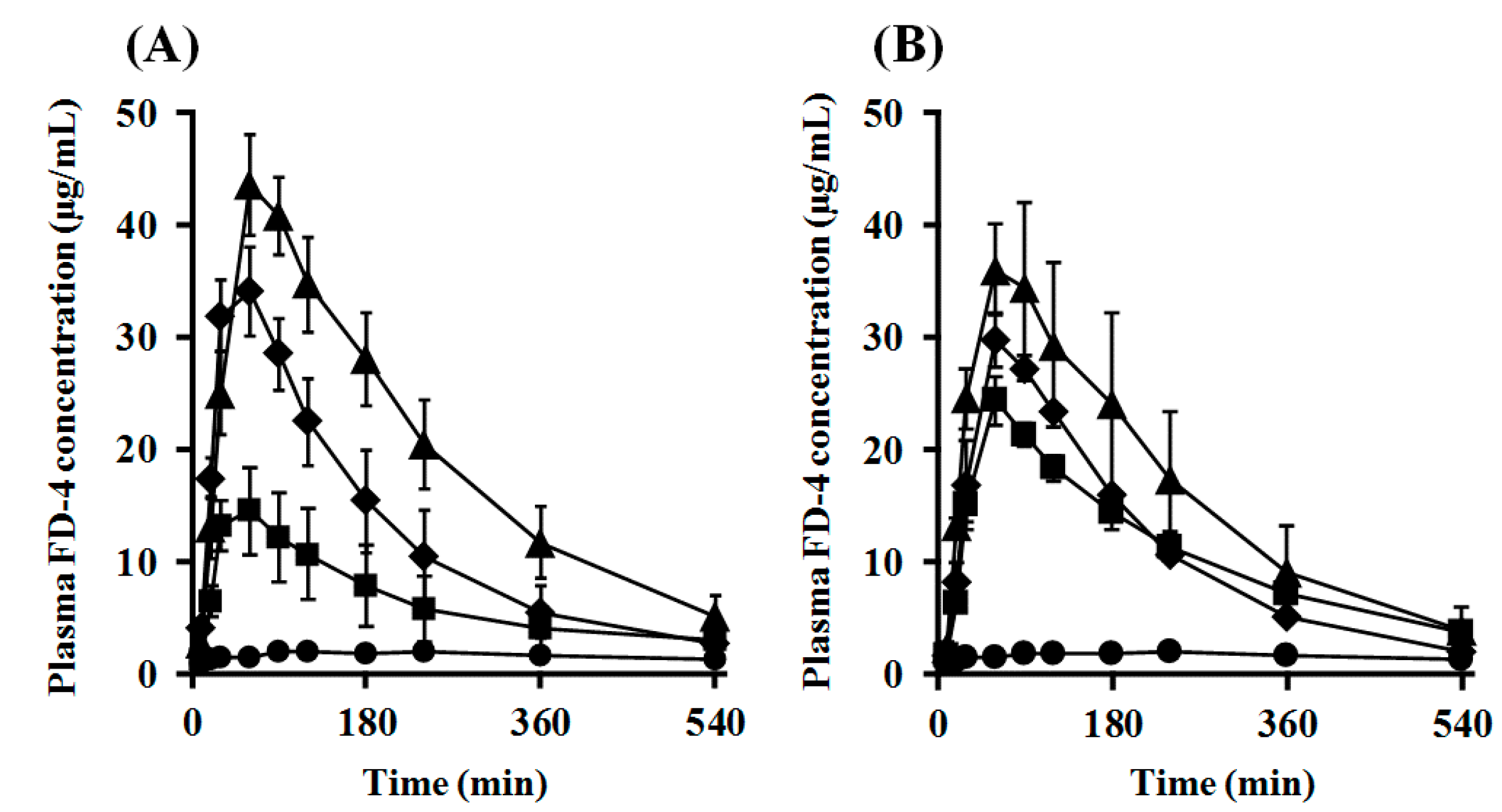
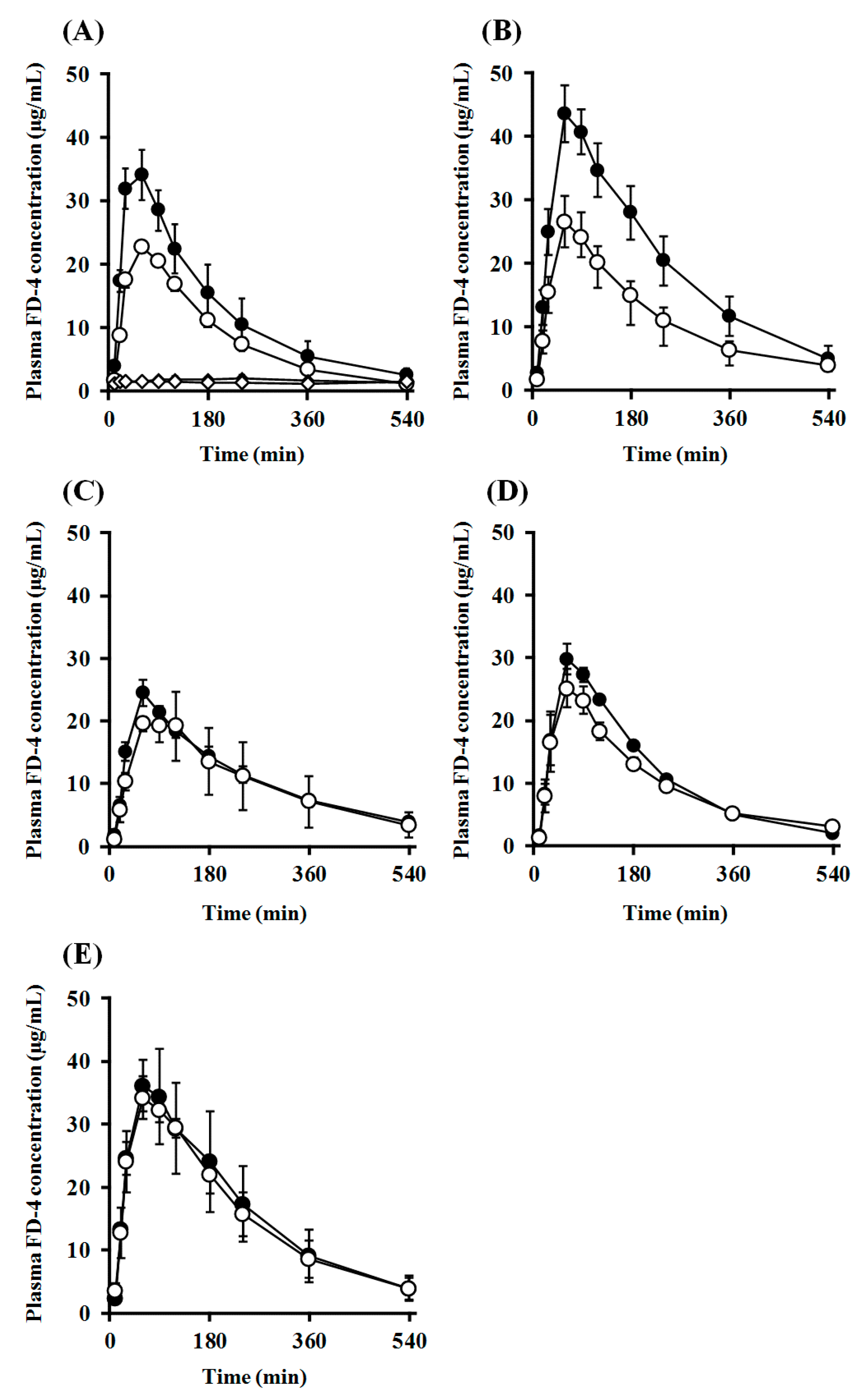
2.7. Impact of PEGylation of PLO on Intranasal Retentivity and Absorption-Enhancing Effect
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Antibodies
4.3. Cell Culture
4.4. Synthesis Procedure of PEG-PLO
4.5. Determination of PEGylation Ratio
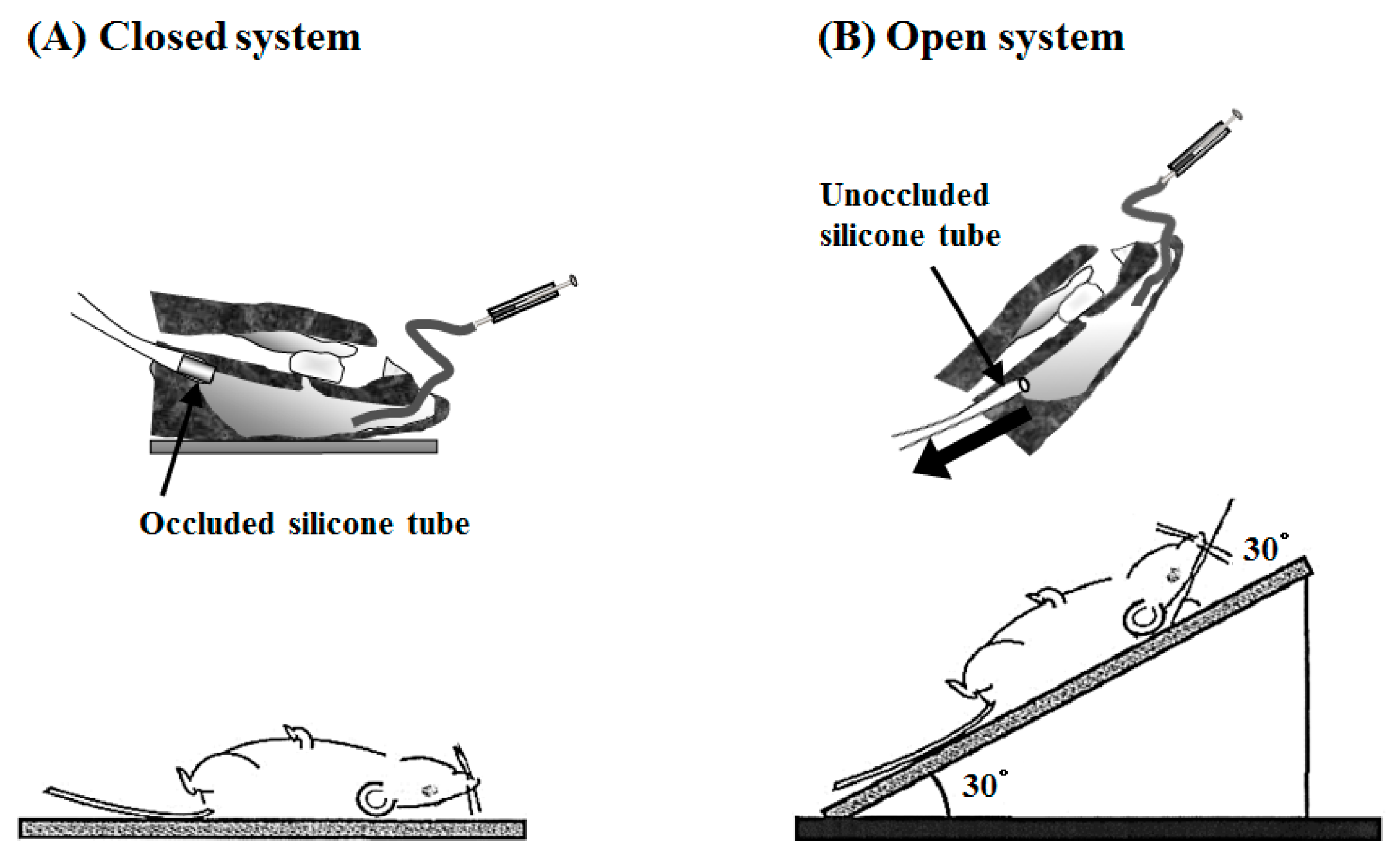
4.6. In Vitro Inclined Plate Test
4.7. Determination of Gt
4.8. FD-4 Permeation Study
4.9. MTT Assay
4.10. Immunofluorescence Microscopic Imaging of TJ Proteins
4.11. Animals
4.12. Intravenous (i.v.) Injection Study
4.13. Intranasal (i.n.) Administration Study
4.14. Pharmacokinetic Analysis
4.15. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- McMartin, C.; Hutchinson, L.E.F.; Hyde, R.; Peters, G.E. Analysis of structural requirements for the absorption of drug and macromolecules from the nasal cavity. J. Pharm. Sci. 1987, 76, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Kubo, H.; Natsume, H.; Sugibayashi, K.; Morimoto, Y.; Yamashita, S. The structural barrier of absorptive mucosae: Site difference of the permeability of fluorescein isothiocyanate-labelled dextran in rabbits. Biopharm. Drug Dispos. 1993, 14, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Natsume, H.; Satoh, I.; Ohtake, K.; Yamaguchi, M.; Kobayashi, D.; Sugibayashi, K.; Morimoto, Y. Effect of poly-l-arginine on the nasal absorption of FITC-dextran of different molecular weights and recombinant human granulocyte colony-stimulating factor (rhG-CSF) in rats. Int. J. Pharm. 2001, 226, 127–138. [Google Scholar] [CrossRef]
- Natsume, H.; Iwata, S.; Ohtake, K.; Miyamoto, M.; Yamaguchi, M.; Hosoya, K.; Kobayashi, D.; Sugibayashi, K.; Morimoto, Y. Screening of cationic compounds as an absorption enhancer for nasal drug delivery. Int. J. Pharm. 1999, 185, 1–12. [Google Scholar] [CrossRef]
- Natsume, H.; Hori, S.; Tsukune, T.; Iwata, S.; Miyamoto, M.; Sugibayashi, K.; Morimoto, Y.; Yamaguchi, M. Improvement of pharmacological response after intranasal administration of peptide drugs by poly-l-arginine as a novel absorption enhancer in rats. Drug Deliv. Syst. 1999, 14, 21–25. [Google Scholar] [CrossRef]
- Ohtake, K.; Maeno, T.; Ueda, H.; Natsume, H.; Morimoto, Y. Poly-l-arginine predominantly increases the paracellular permeability of hydrophilic macromolecules across rabbit nasal epithelium in vitro. Pharm. Res. 2003, 20, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, T.; Ohtake, K.; Ichikawa, K.; Uchida, M.; Uchida, H.; Ohshima, S.; Juni, K.; Morimoto, Y.; Natsume, H. Poly-l-arginine-induced internalization of tight junction proteins increases the paracellular permeability of the Caco-2 cell monolayer to hydrophilic macromolecules. Biol. Pharm. Bull. 2013, 36, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, K.; Natsume, H.; Ueda, H.; Morimoto, Y. Analysis of transient and reversible effects of poly-l-arginine on the in vivo nasal absorption of FITC-dextran in rats. J. Control. Release 2002, 82, 263–275. [Google Scholar] [CrossRef]
- Inoue, D.; Furubayashi, T.; Ogawara, K.; Kimura, T.; Higaki, K.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Higashi, Y. In vitro evaluation of nasal mucociliary clearance using excised rat nasal septum. Biol. Pharm. Bull. 2012, 35, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Gizurarson, S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 2015, 38, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, Y.; Yamaki, T.; Uchida, M.; Hatanaka, T.; Kimura, M.; Ogihara, M.; Morimoto, Y.; Natsume, H. Preparation and evaluation of PEGylated poly-l-ornithine complex as a novel absorption enhancer. Biol. Pharm. Bull. 2017, 40, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ananda, K.; Manjula, B.N.; Meng, F.; Acharya, V.N.; Intaglietta, M.; Acharya, S.A. Packing density of the PEG-shell in PEG-albumins: PEGylation induced viscosity and COP are inverse correlate of packing density. Artif. Cells Blood Substit. Immobil. Biotechnol. 2012, 40, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, T.; Kamaguchi, A.; Kawaharada, K.; Masaoka, Y.; Kataoka, M.; Yamashita, S.; Higashi, Y.; Sakane, T. Kinetic model to predict the absorption of nasally applied drugs from in vitro transcellular permeability of drugs. Biol. Pharm. Bull. 2007, 30, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, T.; Kamiya, Y.; Ohtake, K.; Uchida, M.; Seki, T.; Ueda, H.; Kobayashi, J.; Morimoto, Y.; Natsume, H. A mechanism enhancing macromolecule transport through paracellular spaces induced by poly-l-arginine: Poly-l-arginine induces the internalization of tight junction proteins via clathrin-mediated endocytosis. Pharm. Res. 2014, 31, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Ohtake, K.; Maeno, T.; Uchida, M.; Uchida, H.; Kobayashi, J.; Natsume, H. Relationship between calcein disposition in nasal cavity and blood concentration after intranasal administration of viscous calcein-loaded starch solution in rats. J. Pharm. Sci. Technol. Jpn. 2009, 69, 219–227. [Google Scholar]
- Sivaramakrishnan, M.; Kashyap, A.S.; Amrein, B.; Saenger, S.; Meier, S.; Staudenmaier, C.; Upton, Z.; Metzger, F. PEGylation of lysine residues reduces the pro-migratory activity of IGF-I. Biochim. Biophys. Acta 2013, 1830, 4734–4742. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Tak, K.K.; Park, M.O.; Lee, J.T.; Woo, B.H.; Yoo, S.D.; Lee, H.S.; DeLuca, P.P. Preparation and characterization of polyethylene-glycol-modified salmon calcitonins. Pharm. Dev. Technol. 1999, 4, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Leobandung, W.; Foss, A.; Peppas, N.A. Molecular aspects of muco- and bioadhesion: Tethered structures and site-specific surfaces. J. Control. Release 2000, 65, 63–71. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Controlled synthesis of N,N,N-trimethyl chitosan for modulatedbioadhesion and nasal membrane permeability. Int. J. Biol. Macromol. 2016, 82, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Mazoniene, E.; Joceviciute, S.; Kazlauske, J.; Niemeyer, B.; Liesiene, J. Interaction of cellulose-based cationic polyelectrolytes with mucin. Colloids Surf. B Biointerfaces 2011, 83, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.; Yang, J.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of surface polyethylene glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef] [PubMed]
- Gol, R.M.; Jewrajka, S.K. Facile in situ PEGylation of polyamide thin film composite membranes for improving fouling resistance. J. Membr. Sci. 2014, 455, 271–282. [Google Scholar] [CrossRef]
- Brown, M.D.; Schätzlein, A.; Brownlie, A.; Jack, V.; Wang, W.; Tetley, L.; Gray, A.I.; Uchegbu, I.F. Preliminary characterization of novel amino acid based polymeric vesicles as gene and drug delivery agents. Bioconjug. Chem. 2000, 11, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Castile, J.; Cheng, Y.H.; Simmons, B.; Perelman, M.; Smith, A.; Watts, P. Development of in vitro models to demonstrate the ability of PecSys®, an in situ nasal gelling technology, to reduce nasal run-off and drip. Drug Dev. Ind. Pharm. 2013, 39, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; McClean, S. Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem. Pharmacol. 2006, 71, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Sheth, P.; Elias, B.C.; Rao, R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J. Biol. Chem. 2007, 282, 11487–11498. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Yashiki, T.; Matsuzawa, T.; Mima, H. Absorption of drugs from the nasal mucosa of rat. Int. J. Pharm. 1981, 7, 317–325. [Google Scholar]
| Measurements (%) | C/N (Measurement) | C/N (Theoretical) | Measurement/Theoretical | Measurement Deviation | ||||
| C | H | N | ||||||
| PLO + mPEG-SH | ||||||||
| 1: 5 mixture | 39.79 | 7.20 | 6.74 | 5.90 | 6.02 | 0.98 | 0.99 | |
| 1: 8 mixture | 43.58 | 7.54 | 5.23 | 8.33 | 8.35 | 1.00 | ||
| 1: 11 mixture | 45.05 | 7.87 | 4.24 | 10.63 | 10.68 | 1.00 | ||
| Measurements (%) | C/N from Measurement | Correcting Value | PEG/PLO Ratio | |||||
| C | H | N | ||||||
| PEG-PLO | 48.35 | 8.05 | 5.16 | 9.37 | 9.45 | 8.90 | ||
| C/N ratio (Corrected) (Elemental Analysis) | PEGylation Ratio | Containing PEG per Molecule | Molecular Weight (Approximately) | NH2 Group per 1 kDa (NH2 Contents) | NH2 Contents Before the Pegylation | NH2 Contents Ratio (After/Before PEGylation) | |
|---|---|---|---|---|---|---|---|
| Absorbance Ratio (TNBS Assay) | |||||||
| PEG-PLO | 9.45 | 8.90 | 8–9 | 115,000 | 1.94 | 5.12 | 0.38 |
| 0.374 | 8.96 |
| Route | Dose (mg/kg) | Enhancer | Cmax (μg/mL) | Tmax (min) | AUC0–540 (μg/mL·min) | MRT (min) | F0–540 (%) | ER | MAR (μg/min) | |
|---|---|---|---|---|---|---|---|---|---|---|
| i.v. | 3.3 | - | - | - | 1462.9 ± 222.6 | - | - | - | - | |
| i.n. | 33 | None (Control) | 2.3 ± 0.5 | 187.5 ± 52.5 | 902.4 ± 186.2 | 259.1 ± 16.8 | 6.2 | 1.0 | 2.7 | |
| PLO | ||||||||||
| 0.05% | 16.5 ± 3.3 * | 40.0 ± 10.0 * | 3483.2 ± 1283.4 | 189.5 ± 14.7 * | 23.8 | 3.9 | 28.4 | |||
| 0.25% | 35.9 ± 3.5 * | 60.0 ± 9.5 * | 6667.6 ± 1361.1 * | 153.8 ± 14.8 * | 45.6 | 7.4 | 67.3 | |||
| 0.50% | 44.0 ± 4.3 * | 82.5 ± 14.4 | 10470.4 ± 1522.8 * | 186.8 ± 14.1 * | 71.6 | 11.6 | 56.0 | |||
| PEG-PLO | ||||||||||
| 0.50% | 24.4 ± 2.1 * | 60.0 ± 0.0 * | 5906.6 ± 493.8 * | 201.4 ± 4.7 * | 40.4 | 6.5 | 32.9 | |||
| 1.00% | 29.9 ± 2.3 * | 67.5 ± 7.5 * | 6064.3 ± 332.7 * | 171.7 ± 3.5 * | 41.5 | 6.7 | 38.4 | |||
| 2.00% | 38.6 ± 5.8 * | 70.0 ± 10.1 | 8813.2 ± 2463.9 * | 173.9 ± 18.4 * | 60.2 | 9.8 | 52.7 | |||
| Enhancer | Cmax (μg/mL) | Tmax (min) | AUC0–540 (μg/mL·min) | MRT (min) | F0–540 (%) | ER | MAR (μg/min) | Fo/c (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Closed system | ||||||||||
| None (Control) | 2.3 ± 0.5 | 187.5 ± 52.5 | 902.4 ± 186.2 | 259.1 ± 16.8 | 6.2 | 1.0 | 2.7 | |||
| PLO | 0.25% | 35.9 ± 3.5 | 60.0 ± 9.5 | 6667.6 ± 1361.1 | 153.8 ± 14.8 | 45.6 | 7.4 | 67.3 | ||
| 0.50% | 44.0 ± 4.3 | 82.5 ± 14.4 | 10470.4 ± 1522.8 | 186.8 ± 14.1 | 71.6 | 11.6 | 56.0 | |||
| PEG-PLO | 0.50% | 24.4 ± 2.1 | 60.0 ± 0.0 | 5906.6 ± 493.2 | 201.4 ± 4.7 | 40.4 | 6.5 | 32.9 | ||
| 1.00% | 29.9 ± 2.3 | 67.5 ± 7.5 | 6064.3 ± 332.7 | 171.7 ± 3.5 | 41.5 | 6.7 | 38.4 | |||
| 2.00% | 38.6 ± 5.8 | 70.0 ± 10.0 | 8813.2 ± 2463.9 | 173.9 ± 18.4 | 60.2 | 9.8 | 52.7 | |||
| Open system | ||||||||||
| None (Control) | 1.8 ± 0.5 | 80.0 ± 36.7 | 710.6 ± 135.6 | 269.9 ± 12.9 | 4.9 | 1.0 | 3.6 | 78.7 | ||
| PLO | 0.25% | 22.7 ± 0.9 * | 60.0 ± 0.0 | 4448.3 ± 336.5 | 159.8 ± 7.0 | 30.4 | 6.3 | 37.7 | 66.7 | |
| 0.50% | 26.5 ± 4.3 * | 60.0 ± 0.0 | 6020.9 ± 1009.5 | 189.9 ± 8.8 | 41.2 | 8.5 | 34.1 | 57.5 | ||
| PEG-PLO | 0.50% | 22.6 ± 3.8 | 80.0 ± 20.0 | 5486.5 ± 1904.8 | 190.7 ± 23.1 | 37.5 | 7.7 | 25.0 | 92.9 | |
| 1.00% | 25.4 ± 3.0 | 60.0 ± 0.0 | 5416.5 ± 348.6 | 186.9 ± 11.0 | 37.0 | 7.6 | 36.1 | 89.3 | ||
| 2.00% | 35.3 ± 2.4 | 80.0 ± 20.0 | 8328.3 ± 935.5 | 182.0 ± 20.9 | 56.9 | 11.7 | 50.7 | 94.5 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiya, Y.; Yamaki, T.; Omori, S.; Uchida, M.; Ohtake, K.; Kimura, M.; Yamazaki, H.; Natsume, H. Improved Intranasal Retentivity and Transnasal Absorption Enhancement by PEGylated Poly-l-ornithine. Pharmaceuticals 2018, 11, 9. https://doi.org/10.3390/ph11010009
Kamiya Y, Yamaki T, Omori S, Uchida M, Ohtake K, Kimura M, Yamazaki H, Natsume H. Improved Intranasal Retentivity and Transnasal Absorption Enhancement by PEGylated Poly-l-ornithine. Pharmaceuticals. 2018; 11(1):9. https://doi.org/10.3390/ph11010009
Chicago/Turabian StyleKamiya, Yusuke, Tsutomu Yamaki, Shigehiro Omori, Masaki Uchida, Kazuo Ohtake, Mitsutoshi Kimura, Hiroshi Yamazaki, and Hideshi Natsume. 2018. "Improved Intranasal Retentivity and Transnasal Absorption Enhancement by PEGylated Poly-l-ornithine" Pharmaceuticals 11, no. 1: 9. https://doi.org/10.3390/ph11010009






