Effect of Different Metal Ions on the Biological Properties of Cefadroxil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Uptake and transport studies
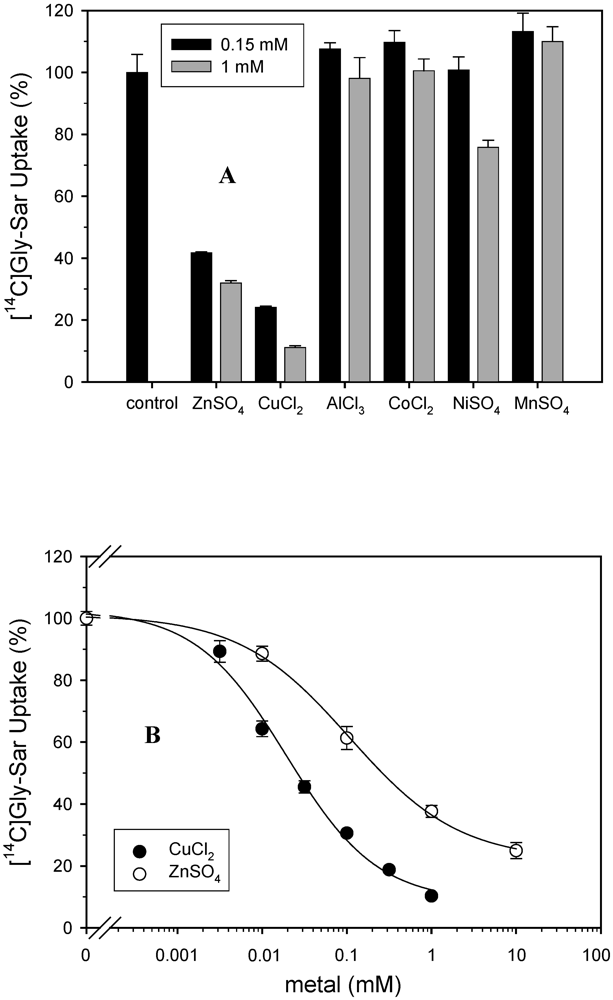
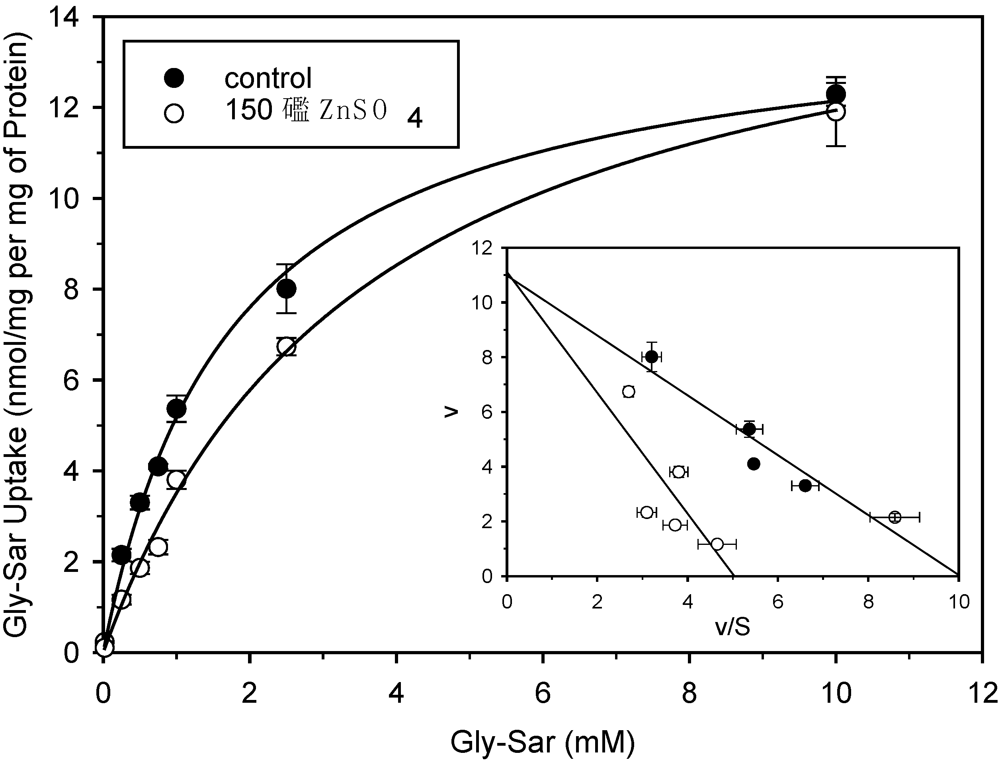
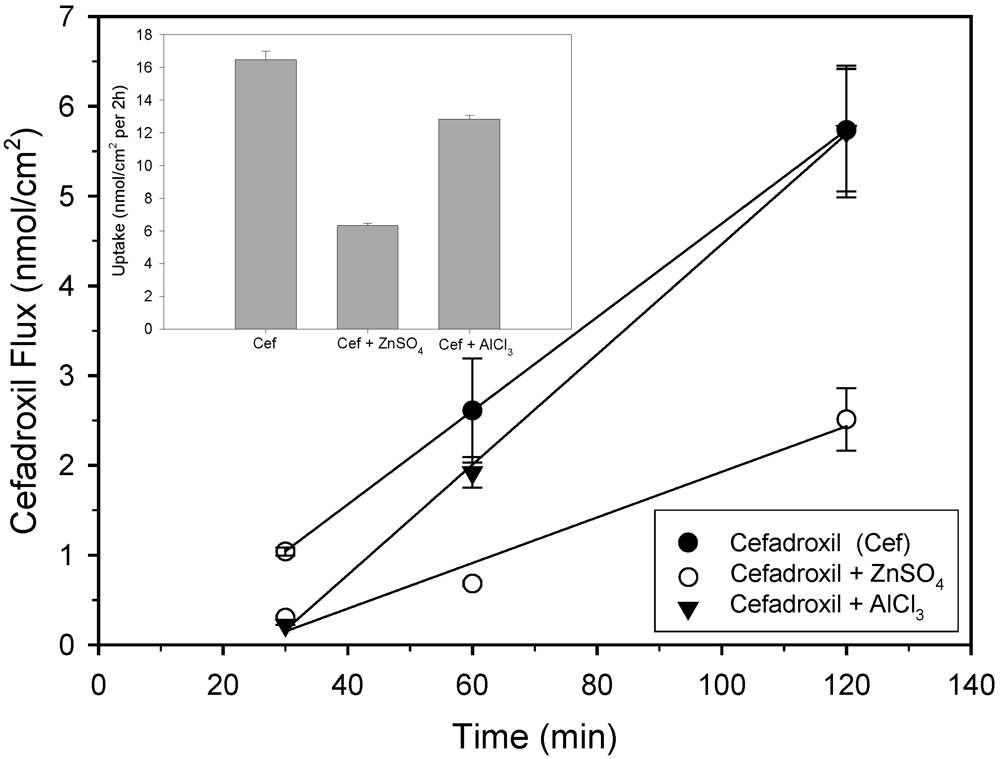
2.2. Antibacterial activity tests
| Bacteria | Inhibition zone (mm) | |||
|---|---|---|---|---|
| Cefadroxil | Cef-Zn | Cef-Cu | Cef-Al | |
| E. coli | 11.5 ± 0.4 | 12.2 ± 0.3 | 9.2 ± 0.4 | 9.7 ± 0.3 |
| S. carnosus | 32.3 ± 0.8 | 33.2 ± 0.8 | 33.3 + 0.9 | 32.5 ± 1.4 |
| P. vulgaris | 0.0 | 0.0 | 0.0 | 0.0 |
| B. subtilis | 33.2 ± 0.6 | 33.8 ± 0.6 | 34.0 ± 0.6 | 34,0 ± 0.6 |
| P. putida | 0.0 | 0.0 | 0.0 | 0.0 |
| M. luteus | 31.0 ± 0.7 | 32.8 ± 0.3 | 32.7 ± 0.4 | 32.7 ± 0.4 |
3. Experimental
3.1. Materials
3.2. Caco-2 cell culture and uptake assessments
3.3. HPLC analysis
3.4. Antimicrobial activity test
3.5. Calculations and Statistics
4. Conclusion
Acknowledgements
References and Notes
- Zayed, M.A.; Abdallah, S.M. Synthesis, characterization and electronic spectra of cefadroxil complexes of d-block elements. Spectrochim. Acta Part A 2004, 60, 2215–2224. [Google Scholar]
- Mrozek-Lyszczek, R. Thermal investigations of cefadroxil complexes with transition metals. Coupled TG-DSC and TG-FTIR techniques. J. Thermal Anal. Calorim. 2004, 78, 473–486. [Google Scholar] [CrossRef]
- Auda, S.H.; Mrestani, Y.; Ahmed, A.M.S.; Neubert, R.H.H. Characterization of the interaction of cefadroxil with different metal ions using capillary electrophoresis. Electrophoresis 2009, 30, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, M.; Knütter, I.; Bosse-Doenecke, E. Pharmaceutical and pharmacological importance of peptide transporters. J. Pharm. Pharmacol. 2008, 60, 543–585. [Google Scholar]
- Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004, 66, 361–384. [Google Scholar]
- Daniel, H.; Boll, M.; Wenzel, U. Physiological importance and characteristics of peptide transport in intestinal epithelial cells. Eur. Assoc. Anim. Prod. 1994, 80, 1–7. [Google Scholar]
- Rubio-Aliaga, I.; Daniel, H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol. Sci. 2002, 23, 434–440. [Google Scholar]
- Daniel, H.; Adibi, S.A. Selective effect of zinc on uphill transport of oligopeptides into kidney brush border membrane vesicles. FASEB J. 1995, 9, 1112–1117. [Google Scholar]
- Bretschneider, B.; Brandsch, M.; Neubert, R. Intestinal transport of β-lactam antibiotics: Analysis of the affinity at H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux. Pharm. Res. 1999, 16, 55–61. [Google Scholar]
- Wenzel, U.; Gebert, I.; Weintraut, H.; Weber, W.; Clauss, W.; Daniel, H. Transport characteristics of differently charged cephalosporin antibiotics in oocytes expressing the cloned intestinal peptide transporter PepT1 and in human intestinal Caco-2 cells. J. Pharmacol. Exp. Ther. 1996, 277, 831–839. [Google Scholar]
- Boll, M.; Herget, M.; Wagener, M.; Weber, W.M.; Markovich, D.; Biber, J.; Clauss, W.; Murer, H.; Daniel, H. Expression cloning and functional characterization of the kidney cortex high-affinity proton-coupled peptide transporter. Pro. Nati. Acad. Sci. USA 1996, 93, 284–289. [Google Scholar]
- Okamura, M.; Terada, T.; Katsura, T.; Saito, H.; Inui, K. Inhibitory effect of zinc on PEPT1-mediated transport of glycylsarcosine and β-lactam antibiotics in human intestinal cell line Caco-2. Pharm. Res. 2003, 20, 1389–1393. [Google Scholar]
- Tacnet, F.; Lauthier, F.; Ripoche, P. Mechanisms of zinc transport into pig small intestine brush-border membrane vesicles. J. Physiol. 1993, 465, 57–72. [Google Scholar]
- Ueno, K.; Tanaka, K.; Tsujimura, K.; Morishima, Y.; Iwashige, H.; Yamazaki, K.; Nakata, I. Impairment of cefdinir absorption by iron ion. Clin. Pharmacol. Ther. 1993, 54, 473–475. [Google Scholar]
- Rodriguez-Yoldi, M.C.; Mesonero, J.E.; Rodriguez-Yoldi, M.J. Action of zinc on enzymatic digestion and intestinal transport of sugar in the rabbit. Res. Vet. Sci. 1994, 57, 15–20. [Google Scholar]
- Glahn, R.P.; Van Campen, D.R. Iron uptake is enhanced in Caco-2 cellmonolayers by cycteine and reduced cysteinyl glycine. J. Nutr. 1997, 127, 642–647. [Google Scholar]
- Watkins, D.W.; Chenu, C.; Ripoche, P. Zinc inhibition of glucose uptake in brush border membrane vesicles from pig small intestine. Pflugers Arch. 1989, 415, 165–171. [Google Scholar]
- Rodriguez-Yoldi, M.C.; Mesonero, J.E.; Rodriguez-Yoldi, M.J. Effect of zinc on aminopeptidase N activity and L-threonine transport in rabbit jejunum. Biol. Trace Elem. Res. 1996, 53, 213–223. [Google Scholar]
- Steinhardt, H.J.; Adibi, S.A. Interaction between transport of zinc and othersolutes in human intestine. Am. J. Physiol. 1984, 247, 176–182. [Google Scholar]
- Anacona, J.R.; Rodriguez, I. Synthesis and antibacterial activity of cephalexin metal complexes. J. Coord. Chem. 2004, 57, 1263–1269. [Google Scholar] [CrossRef]
- Anacona, J.R.; Alvarez, P. Synthesis and antibacterial activity of metal complexes of cefazolin. Trans. Met. Chem. 2002, 27, 856–860. [Google Scholar]
- Anacona, J.R.; Acosta, F. Synthesis and antibacterial activity of cephradine metal complexes. J. Coord. Chem. 2005, 59, 621–627. [Google Scholar]
- Chohan, Z.H. Synthesis of cobalt(II) and nickel(II) complexes of Ceclor (Cefaclor) and preliminary experiments on their antibacterial character. Chem. Pharm. Bull. 1991, 39, 1578–1580. [Google Scholar]
- Iqbal, M.S.; Ahmed, A.R.; Sabir, M.; Asad, S.M. Preparation, characterization and biological evaluation of copper(II) and zinc(II) complexes with cephalexin. J. Pharm. Pharmacol. 1999, 51, 371–375. [Google Scholar]
- Brandsch, M.; Miyamoto, Y.; Ganapathy, V.; Leibach, F.H. Expression and protein kinase C-dependent regulation of peptide/H+ co-transport system in the Caco-2 human colon carcinoma cell line. Biochem. J. 1994, 1, 253–260. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Auda, S.H.; Knütter, I.; Bretschneider, B.; Brandsch, M.; Mrestani, Y.; Große, C.; Neubert, R.H.H. Effect of Different Metal Ions on the Biological Properties of Cefadroxil. Pharmaceuticals 2009, 2, 184-193. https://doi.org/10.3390/ph2030184
Auda SH, Knütter I, Bretschneider B, Brandsch M, Mrestani Y, Große C, Neubert RHH. Effect of Different Metal Ions on the Biological Properties of Cefadroxil. Pharmaceuticals. 2009; 2(3):184-193. https://doi.org/10.3390/ph2030184
Chicago/Turabian StyleAuda, Sayed H., Ilka Knütter, Beate Bretschneider, Matthias Brandsch, Yahya Mrestani, Cornelia Große, and Reinhard H. H. Neubert. 2009. "Effect of Different Metal Ions on the Biological Properties of Cefadroxil" Pharmaceuticals 2, no. 3: 184-193. https://doi.org/10.3390/ph2030184




