2.1. Ca2+ Imaging of Ventricular Myocytes
IpTxa’s ability to induce subconducting states in single channels and to enhance binding of [
3H]ryanodine to SR vesicles distinguish it as a RyR agonist. Furthermore, IpTxa’s high sequence identity with MCa suggests that the toxin may also be a cell-penetrating peptide. We therefore designed experiments to both investigate the effects of IpTxa on calcium signaling in intact cardiomyocytes, and to demonstrate its cell-penetrating capabilities. Ventricular cardiomyocytes were enzymatically digested, loaded with the Ca
2+ indicator Fluo-3, and placed into the perfusion chamber of a Zeiss confocal microscope (see Experimental Methods). Cells were field-stimulated under Normal Tyrode (NT) solution, and Ca
2+ transients were recorded in line-scan mode (control). Perfusion was then changed to NT + IpTxa (in the range of 100 nM-30 µM), and transients were recorded in the presence of toxin (+IpTxa). We hypothesized that if IpTxa penetrates cardiomyocytes to reach its intracellular target, it would alter the shape of [Ca
2+]
i transients elicited with field-stimulation. As expected, changes in [Ca
2+]
i transients were evident within seconds of perfusion with the toxin. Perfusion of IpTxa at high concentrations (30 µM) resulted in the complete cessation of [Ca
2+]
i transients (
Figure 1). For this experiment, cells were paced at 0.5 Hz under NT solution (see Experimental Methods). Cells were scanned first without IpTxa (control transients,
Figure 1A), and then IpTxa was perfused and a second scan was taken immediately afterward (
Figure 1B).
At lower concentrations (100-300 nM), IpTxa perfusion elicited several discernible stimulatory effects in most cells. The most commonly observed response to perfusion with these IpTxa concentrations was a significant increase in the amplitude of the [Ca
2+]
i transient, which was followed by a gradual diminution in transient amplitude until arriving at a new, lower steady-state (
Figure 2). In similar [Ca
2+]
i transient experiments, Eisner’s group found that 0.5 mM caffeine, a RyR agonist, elicited a brief increase in the amplitude of the Ca
2+ transient, followed by a steady decline in amplitude to a new steady-state at a lower amplitude than in control transients [
8]. They attributed the observed effects to partial depletion of SR Ca
2+. The biphasic response elicited by IpTxa also closely mirrors that recently reported for Hadrucalcin, a recent addition to the calcin family, isolated from
Hadrurus gertschi [
9].
Figure 1.
Perfusion of intact ventricular cardiomyocytes with a high concentration (30 µM) of IpTxa causes immediate cessation of [Ca2+]i transients. (A) line-scan image (top) and associated fluorescence plot (bottom) of a field-stimulated mouse ventricular cardiomyocyte loaded with the Ca2+ indicator Fluo-4 and perfused constantly with normal Tyrodes solution in the absence of IpTxa (control). (B) The same cell ~1 min after perfusion with the same Tyrodes solution, but containing 30 µM IpTxa. The protocol was repeated four times and the result below is representative of three experiments.
Figure 1.
Perfusion of intact ventricular cardiomyocytes with a high concentration (30 µM) of IpTxa causes immediate cessation of [Ca2+]i transients. (A) line-scan image (top) and associated fluorescence plot (bottom) of a field-stimulated mouse ventricular cardiomyocyte loaded with the Ca2+ indicator Fluo-4 and perfused constantly with normal Tyrodes solution in the absence of IpTxa (control). (B) The same cell ~1 min after perfusion with the same Tyrodes solution, but containing 30 µM IpTxa. The protocol was repeated four times and the result below is representative of three experiments.
Figure 2.
Perfusing intact cardiomyocytes with 100 nM IpTxa produced an increase in the amplitude of the [Ca2+]i transient followed by a gradual decrease to a new steady-state. Line-scan image (top) and associated fluorescence plot (bottom) of a field-stimulated mouse ventricular cardiomyocyte loaded with the Ca2+ indicator Fluo-4 and perfused constantly with normal Tyrodes solution in the absence of IpTxa. At the time indicated by the arrow, IpTxa (100 nM) or caffeine (10 mM) was perfused onto the cell. The protocol was repeated 36 times and the result below is representative of 12 experiments.
Figure 2.
Perfusing intact cardiomyocytes with 100 nM IpTxa produced an increase in the amplitude of the [Ca2+]i transient followed by a gradual decrease to a new steady-state. Line-scan image (top) and associated fluorescence plot (bottom) of a field-stimulated mouse ventricular cardiomyocyte loaded with the Ca2+ indicator Fluo-4 and perfused constantly with normal Tyrodes solution in the absence of IpTxa. At the time indicated by the arrow, IpTxa (100 nM) or caffeine (10 mM) was perfused onto the cell. The protocol was repeated 36 times and the result below is representative of 12 experiments.
In addition to the above response, we observed elevated diastolic [Ca
2+]
i in some cells perfused with 100 nM to 3 µM IpTxa (
Figure 3). This effect could be indicative of Ca
2+ leak through RyRs, a possibility that we are exploring in a separate study. Finally, at all concentrations of IpTxa tested, cells frequently exhibited contractions that were discordant with field stimulation. These unsolicited contractions presumably result from a critical amount of Ca
2+ leak through IpTxa-activated RyRs that is capable of propagating to neighboring release sites and activate IpTxa-free RyRs. Any of these Ca
2+ release waves or leaks could potentially be a substrate for arrhythmias due to activation of the electrogenic sodium-calcium exchanger (NCX), which is known to induce delayed afterdepolarizations (DADs). However, the exact mechanism by which IpTxa elicits cessation of contractions, subtle Ca
2+ leak or discrete Ca
2+ waves requires further investigation.
Figure 3.
Perfusing 3 µM IpTxa increased diastolic [Ca]i and produced irregular [Ca2+]i transients. Line-scan image (top) and associated fluorescence plot (bottom) of a field-stimulated mouse ventricular cardiomyocyte loaded with the Ca2+ indicator Fluo-4 and perfused constantly with normal Tyrodes solution in the absence of IpTxa. At the time indicated by the arrow, IpTxa (3 μM) or caffeine (10 mM) was perfused onto the cell. The protocol was repeated 36 times and the result below is representative of three experiments.
Figure 3.
Perfusing 3 µM IpTxa increased diastolic [Ca]i and produced irregular [Ca2+]i transients. Line-scan image (top) and associated fluorescence plot (bottom) of a field-stimulated mouse ventricular cardiomyocyte loaded with the Ca2+ indicator Fluo-4 and perfused constantly with normal Tyrodes solution in the absence of IpTxa. At the time indicated by the arrow, IpTxa (3 μM) or caffeine (10 mM) was perfused onto the cell. The protocol was repeated 36 times and the result below is representative of three experiments.
2.2 Modification of IpTxa
Until recently, it was presumed that peptide toxins were incapable of crossing the plasma membrane and therefore to not recognize intracellular targets. The rapid onset of noticeable effects after IpTxa perfusion on intact cardiomyocytes strongly suggests that IpTxa crosses the cell membrane to reach its intracellular target rather than mimicking a membrane-embedded receptor, as was previously suggested [
10]. Calcins, like other cell-penetrating peptides, are extremely basic peptides, with a net charge of at least +7 at physiological pH, which suggests that they, too, could permeate membranes [
11,
12]. It is also known that highly basic peptides translocate to the cytosol by locally perturbing the integrity of the lipid bilayer [
13]. Translocation across the membrane could logically be the result of the interaction of the positive charges of the basic residues with negatively-charged polar heads of fatty acids of the plasma membrane. Indeed, in a recent series of elegant papers, MCa has been shown to translocate across cell membranes in a variety of cell types by virtue of an interaction of heparin and heparin sulfate and by macropinocytosis [
14,
15]. A linear analogue of MCa was shown to retain its cell-penetrating capability, though its pharmacological activity on RyRs was abolished [
3].
In order to visualize the translocation of IpTxa into the cytosol of intact cells, we synthesized a fluorescently-labeled IpTxa derivative. It has been reported that Lysine 22 (K22) together with other basic residues (R23, R24, R31 and R33) form a critical domain of IpTxa that acts as a putative binding site and interacts with a region of the RyR [
7,
10,
16]. In order to produce fluorescent derivatives of IpTx–a with minimum alteration of conformational or biological properties, we used a cyclic imidoester, 2-iminothiolane, to introduce a unique sulfhydryl group at the N-terminus without disrupting the critical binding site of the toxin. This addition made it possible to introduce subsequent modifications with fluorescent maleimide compounds. We used pH 7.0 in all reactions in order to ensure modification of the N-terminal group rather than ε-amino of the lysine residues. The mono-derivative was prepared using the labeling procedure described above. We then separated mono-2IT-IpTxa from unmodified toxin with HPLC and verified it with mass spectrometry (see Methods). 2IT-IpTxa was modified with fluorescent maleimide-Alexa-546, then separated and verified as above. The mono-modified derivative, Alexa-546-IpTxa, was used for subsequent chemical and biological characterization.
2.3. Properties of Modified Imperatoxin A. Biological Activity and Fluorescent Properties
Alexa-Fluor-546-derivatized-IpTxa (Alexa-IpTxa) retained the spectral properties of the parent fluorophore (
Figure 4).
Figure 4.
Absorption (solid line) and emission (broken line) spectra of Alexa-Fluor 546 (A) and Alexa-IpTxa (B). Unmodified IpTxa (not shown) lacks emission properties.
Figure 4.
Absorption (solid line) and emission (broken line) spectra of Alexa-Fluor 546 (A) and Alexa-IpTxa (B). Unmodified IpTxa (not shown) lacks emission properties.
In addition, fluorescent IpTxa, like the parent molecule, enhanced the binding of [
3H]ryanodine, although higher concentrations were needed to obtain the same half-maximal effective concentration (EC
50). As reported previously, EC
50 was 10 nM for native IpTxa, whereas Alexa-IpTxa exhibited an EC
50 of 75 nM. Thus, modified IpTxa shows excellent activation of RyRs, which is only 7.5-fold lower than the native toxin. This result is impressive in light of previous chemical modification studies using scorpion toxins, which have indicated that modifications of amino groups lead to a loss of toxic activity [
17]. This activity may be preserved, however, through guanidation of amino groups and limited iodination of tyrosine. These results suggest that the protonation state of the toxin is critical and that successful modification and introduction of fluorescent fluorophores depends on the preservation of the peptide’s net charge [
21]. This improves the likelihood that local side chain interactions will be maintained, which in turn favors the conservation of the toxin’s tertiary structure and toxic activity.
Figure 5.
Dose-dependent activation of [3H]-ryanodine binding by native IpTxa and Alexa-IpTxa. Skeletal SR vesicles were incubated with 7 nM [3H]ryanodine in the absence (control 100%) and the indicated concentration of native (B) or modified IpTxa (A) The binding reaction was carried out for 90 min at 36˚C in medium containing 0.2 M KCl, 10 mM Na-HEPES (pH 7.2) and 10 μM CaCl2. Free and bound ligand were separated by rapid filtration as described in methods. Results are the mean ± S.D. of n=4 independent experiments.
Figure 5.
Dose-dependent activation of [3H]-ryanodine binding by native IpTxa and Alexa-IpTxa. Skeletal SR vesicles were incubated with 7 nM [3H]ryanodine in the absence (control 100%) and the indicated concentration of native (B) or modified IpTxa (A) The binding reaction was carried out for 90 min at 36˚C in medium containing 0.2 M KCl, 10 mM Na-HEPES (pH 7.2) and 10 μM CaCl2. Free and bound ligand were separated by rapid filtration as described in methods. Results are the mean ± S.D. of n=4 independent experiments.
2.4 Penetration of Intact Cardiomyocytes by Alexa-IpTxa
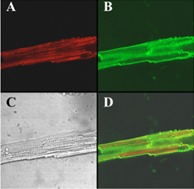
To visualize the cell-penetrating properties of Alexa-IpTxa in intact cells, ventricular cardiomyocytes were enzymatically isolated and incubated with 5 μM Alexa-IpTxa and Alexa 488-Wheat Germ Agglutinin (10 μg/mL) for 30 min at room temperature (see Experimental Methods). The distribution of Alexa-546-IpTxa (red fluorescence) and WAG-Alexa-488 (green fluorescence) were monitored in living, unfixed cardiac myocytes.
Figure 6 shows images of a ventricular myocyte after this incubation. The transmitted light image is shown in C, Alexa-IpTxa staining in A, and wheat-germ agglutinin staining in B. Finally, D shows the merged image of A and B. It is clear that Alexa-IpTxa fluorescence localizes in the sarcoplasm of the cell, demonstrating that IpTxa, aside from being a potent and specific RyR activator, can cross the plasma membrane to interact with its target on the sarcoplasmic reticulum.
Figure 6.
Alexa-IpTxa shows an intracellular localization in intact mouse ventricular myocytes. A, Alexa-IpTxa fluorescence; B, Alexa-Fluor-488 WGA; C, transmitted light image; D, merging of A and B images.
Figure 6.
Alexa-IpTxa shows an intracellular localization in intact mouse ventricular myocytes. A, Alexa-IpTxa fluorescence; B, Alexa-Fluor-488 WGA; C, transmitted light image; D, merging of A and B images.
2.5 Kinetics of Cardiomyocyte Penetration by Alexa-IpTxa
We next sought to establish a time-course for the translocation of Alexa-IpTxa across the plasma membrane of intact cardiomyocytes.
Figure 7 shows transmitted light images of ventricular myocytes before (A, C, and E) and after incubation with Alexa-maleimide (B), which retains fluorescence but it is membrane-impermeable (thus serving as negative control for this experiment), or with Alexa-IpTxa (D and F), to verify penetration of the fluorescent derivative of IpTxa. Clear intracellular localization of fluorescence is seen only with Alexa-IpTxa, as early as 10 min after incubation (panel D), but more intensely after 20 min incubation (panel F). In order to refine the time-course of Alexa-IpTxa penetration into cardiomyocytes, we continuously monitored the evolution of fluorescence in 3 regions of interest (ROI) corresponding to the plasma membrane and adjacent cytosol (ROI-1), the bulk cytosol (ROI-2), and the nucleus (ROI-3). A fourth ROI (not shown) was defined to monitor changes in fluorescence of the bath solution. Animations of IpTxa translocation into cells are available online as
supplementary data (URL:
https://www.mdpi.com/1424-8247/3/4/1093/S1).
Figure 7.
Translocation of IpTxa from external to intracellular compartments of intact mouse ventricular myocytes. All images represent the transmitted light image of the cardiomyocytes (C and B also show Fluo-3 fluorescence). A, C, and E were imaged at the beginning of the protocol, before the start of Alexa-IpTxa perfusion. B, Same cell as A, but after 10 minutes of Alexa-maleimide (1 μM) perfusion. D, Same cell as C, but after 10 minutes of Alexa-IpTxa (1 μM) perfusion. F, Same cell as E, but after 30 minutes of Alexa-IpTxa (1 μM) perfusion.
Figure 7.
Translocation of IpTxa from external to intracellular compartments of intact mouse ventricular myocytes. All images represent the transmitted light image of the cardiomyocytes (C and B also show Fluo-3 fluorescence). A, C, and E were imaged at the beginning of the protocol, before the start of Alexa-IpTxa perfusion. B, Same cell as A, but after 10 minutes of Alexa-maleimide (1 μM) perfusion. D, Same cell as C, but after 10 minutes of Alexa-IpTxa (1 μM) perfusion. F, Same cell as E, but after 30 minutes of Alexa-IpTxa (1 μM) perfusion.
Figure 8.
Evolution of Alexa-IpTxa fluorescence in three regions of interest. Blue, ROI-1, corresponding to the cell membrane and adjacent cytoplasm; Red, ROI-2 (bulk cytoplasm); Black, ROI-3 (nucleus).
Figure 8.
Evolution of Alexa-IpTxa fluorescence in three regions of interest. Blue, ROI-1, corresponding to the cell membrane and adjacent cytoplasm; Red, ROI-2 (bulk cytoplasm); Black, ROI-3 (nucleus).
An increase in fluorescence intensity was observed for ROI-1 and ROI-3 <50 seconds after the beginning of perfusion with 1 μM Alexa-IpTxa (
Figure 8). A slower and much smaller increase in intensity was observed for ROI-3 (even after 30 minutes), suggesting that translocation across the plasma membrane into the cytosol (rather than plasma membrane to cytosol, then from cytosol to nucleus) is the more favorable pathway. The fact that fluorescence intensity was greater in all cellular compartments than that observed in the bath is indicative that intact cells act as toxin concentrators, as previously observed for MCa [
7]. In contrast, cells perfused with Alexa-maleimide show only labeling of the membrane.