Lucifensins, the Insect Defensins of Biomedical Importance: The Story behind Maggot Therapy
Abstract
:1. Introduction
2. Insect Defensins
3. Maggot Therapy
4. The Brief History of the Search for Antimicrobial Agents in Medicinal Larvae
5. Lucifensin—The Defensin from L. sericata
5.1. Purification and Sequence Determination
5.2. Synthetic Lucifensin
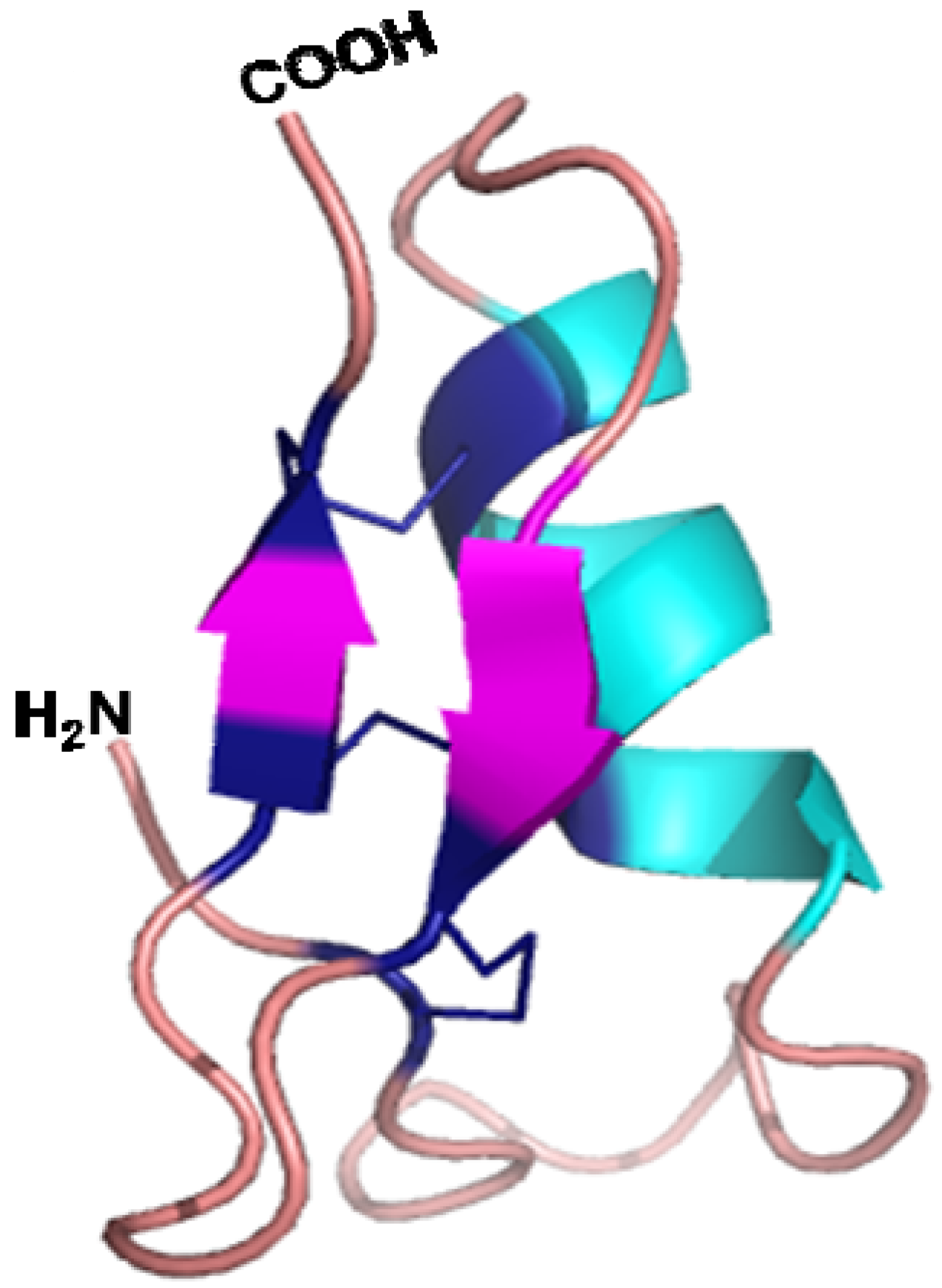
5.3. Three Dimensional Structure and Mode of Action
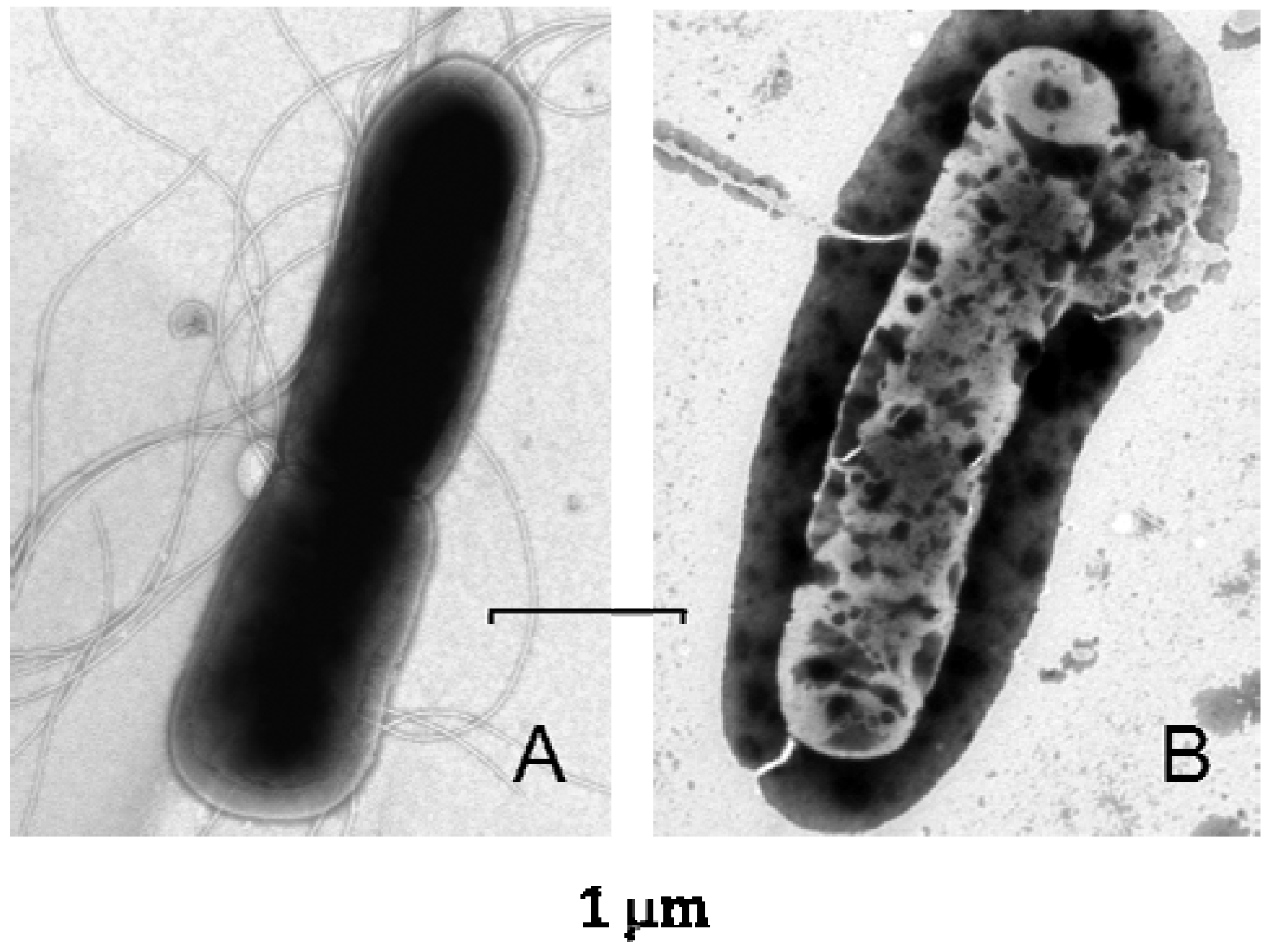
6. Lucifensin II—The Defensin from L. cuprina
7. Molecular Biology Approaches for the Identification of Lucifensin in Medicinal Larvae
8. Lucifensin Released by Maggots to the Wound
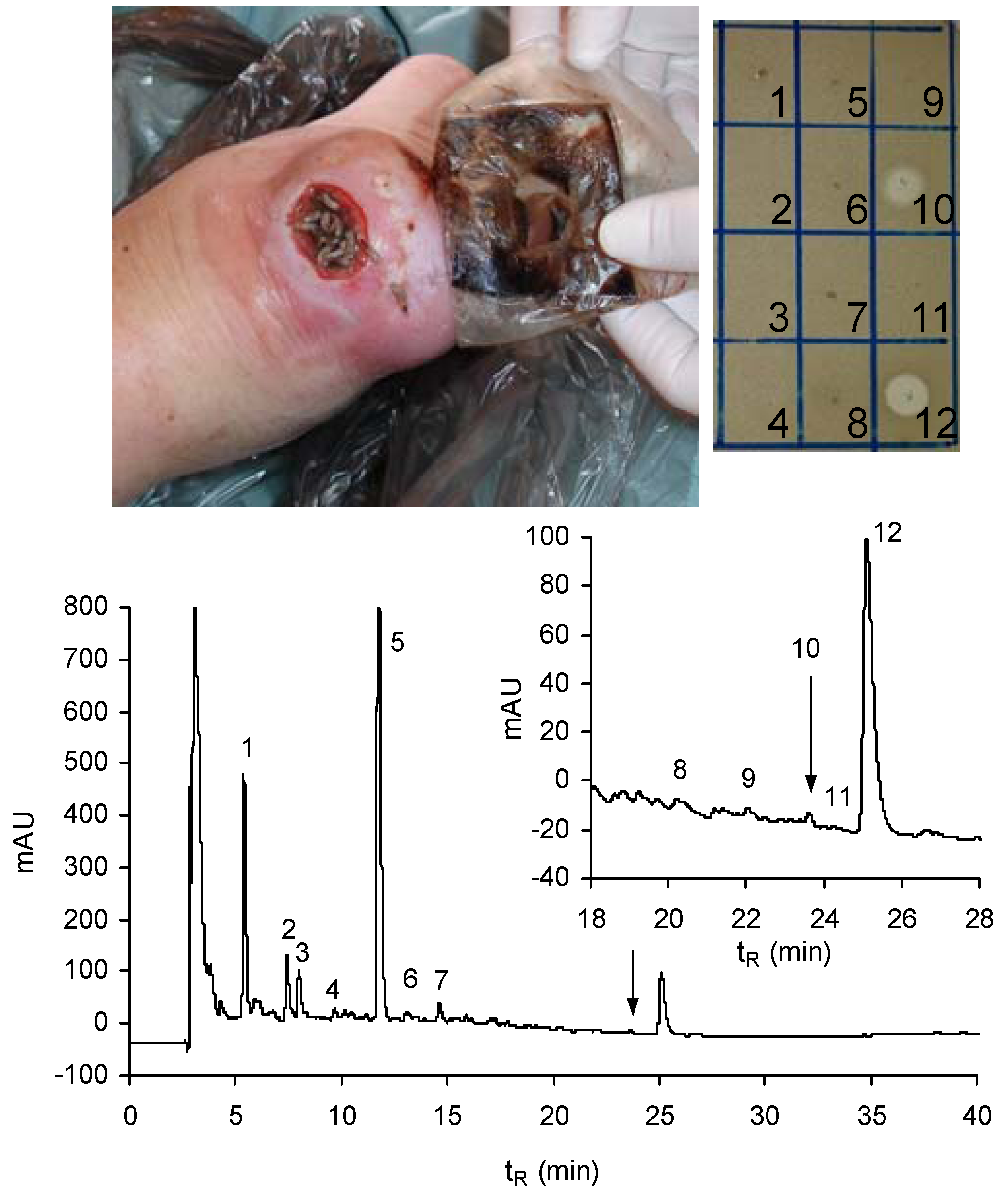
9. Perspectives on the Future of Lucifensins
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Li, X.; Wang, Z. APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009, 37, D933–D937. [Google Scholar] [CrossRef]
- Bulet, P.; Hetru, C.; Dimarcq, J.-L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Brown, K.L.; Hancock, R.E.W. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, α-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Toke, O. Antimicrobial peptides: New candidates in the fight against bacterial infections. Biopolymers 2005, 80, 717–735. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Giuliani, A.; Pirri, G.; Nicoletto, S.F. Antimicrobial peptides: An overview of a promising class of therapeutics. Centr. Eur. J. Biol. 2007, 2, 1–33. [Google Scholar] [CrossRef]
- Čeřovský, V.; Ždárek, J.; Fučík, V.; Monincová, L.; Voburka, Z.; Bém, R. Lucifensin, the long-sought antimicrobial factor of medicinal maggots of the blowfly Lucilia sericata. Cell. Mol. Life Sci. 2010, 67, 455–466. [Google Scholar] [CrossRef]
- El Shazely, B.; Veverka, V.; Fučík, V.; Voburka, Z.; Žďárek, J.; Čeřovský, V. Lucifensin II, a defensin of medicinal maggots of the blowfly Lucilia cuprina (Diptera: Calliphoridae). J. Med. Entomol. 2013, 50, 571–578. [Google Scholar] [CrossRef]
- Hoffmann, J.A.; Hetru, C. Insect defensins: inducible antimicrobial peptides. Immunol. Today 1992, 13, 411–415. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Peptide Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef]
- Sherman, R.A.; Hall, M.J.R.; Thomas, S. Medicinal maggots: An ancient remedy for some contemporary afflictions. Annu. Rev. Entomol. 2000, 45, 55–81. [Google Scholar] [CrossRef]
- Nigam, Y.; Dudley, E.; Bexfield, A.; Bond, A.E.; Evans, J.; James, J. The physiology of wound healing by the medicinal maggot, Lucilia sericata. Adv. Insect Physiol. 2010, 39, 39–81. [Google Scholar] [CrossRef]
- Matsuyama, K.; Natori, S. Purification of three antibacterial proteins from the culture medium of NIH-Sape-4, an embryonic cell line of Sarcophaga peregrina. J. Biol. Chem. 1988, 263, 17112–17116. [Google Scholar]
- Lambert, J.; Keppi, E.; Dimarcq, J.-L.; Wicker, C.; Reichhart, J.-M.; Dunbar, B.; Lepage, P.; Van Dorsselaer, A.; Hoffmann, J.; Forthergill, J.; Hoffmann, D. Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides. Proc. Natl. Acad. Sci. USA 1989, 86, 262–266. [Google Scholar] [CrossRef]
- Lehane, M.J.; Wu, D.; Lehane, S.M. Midgut-specific immune molecules are produced by the blood-sucking insect Stomoxys calcitrans. Proc. Natl. Acad. Sci.USA 1997, 94, 11502–11507. [Google Scholar] [CrossRef]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antimicrobial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar]
- Rees, J.A.; Moniatte, M.; Bulet, P. Novel antimicrobial peptides isolated from a European bumblebee, Bombus pascuorum (Hymenoptera, Apoidea). Insect. Biochem. Molec. Biol. 1997, 27, 413–422. [Google Scholar] [CrossRef]
- Hanzawa, H.; Shimada, I.; Kuzuhara, T.; Komano, H.; Kohda, D.; Inagaki, F.; Natori, S.; Arata, Y. 1H nuclear magnetic resonance study of the solution conformation of an antibacterial protein, sapecin. FEBS Lett. 1990, 269, 413–420. [Google Scholar] [CrossRef]
- Cornet, B.; Bonmatin, J.-M.; Hetru, C.; Hoffmann, J.A.; Ptak, M.; Vovelle, F. Refined three-dimensional solution structure of insect defensin A. Structure 1995, 3, 435–448. [Google Scholar] [CrossRef]
- Landon, C.; Sodano, P.; Hetru, C.; Hoffmann, J.; Ptak, M. Solution structure of drosomycin, the first inducible antifungal protein from insects. Protein Sci. 1997, 6, 1878–1884. [Google Scholar] [CrossRef]
- Mumcuoglu, K. Y.; Miller, J.; Mumcuoglu, M.; Friger, M.; Tarshis, M. Destruction of bacteria in the digestive tract of the maggot of Lucilia sericata (Diptera: Calliphoridae). J. Med. Entomol. 2001, 38, 161–166. [Google Scholar] [CrossRef]
- Parnés, A.; Lagan, K. M. Larval therapy in wound management: A review. Int. J. Clin. Pract. 2007, 61, 488–493. [Google Scholar] [CrossRef]
- Baer, W.S. The treatment of chronic osteomyelitis with the maggots (larva of the blowfly). J. Bone Joint. Surg. 1931, 13, 438. [Google Scholar]
- Simons, S.W. A bactericidal principle in excretions of surgical maggots which destroys important etiological agents of pyogenic infections. J. Bacteriol. 1935, 30, 253–267. [Google Scholar]
- Pavillard, E.R.; Wright, E.A. An antibiotic from maggots. Nature 1957, 180, 916–917. [Google Scholar] [CrossRef]
- Huberman, L.; Gollop, N.; Mumcuoglu, K.Y.; Breuer, E.; Bhusare, S.R.; Shai, Y.; Galun, R. Antibacterial substances of low molecular weight isolated from the blowfly, Lucilia sericata. Med. Vet. Entomol. 2007, 21, 127–131. [Google Scholar] [CrossRef]
- Thomas, S.; Andrews, A.M.; Hay, N.P.; Bourgoise, S. The anti-microbial activity of maggot secretions: results of a preliminary study. J. Tissue Viability 1999, 9, 127–132. [Google Scholar]
- Bexfield, A.; Nigam, Y.; Thomas, S.; Ratcliffe, N.A. Detection and partial characterisation of two antibacterial factors from the excretions/secretions of the medicinal maggot Lucilia sericata and their activity against methicillin-resistant Staphylococcus aureus (MRSA). Microbes Infect. 2004, 6, 1297–1304. [Google Scholar] [CrossRef]
- Bexfield, A.; Bond, A.E.; Roberts, E.C.; Dudley, E.; Nigam, Y.; Thomas, S.; Newton, R.P.; Ratcliffe, N.A. The antibacterial activity against MRSA strains and other bacteria of a <500 Da fraction from maggot excretions/secretions of Lucilia sericata (Diptera: Calliphoridae). Microbes Infect. 2008, 10, 325–333. [Google Scholar] [CrossRef]
- Jaklič, D.; Lapanje, A.; Zupančič, K.; Smrke, D.; Gunde-Cimerman, N. Selective antimicrobial activity of maggots against pathogenic bacteria. J. Med. Microbiol. 2008, 57, 617–625. [Google Scholar] [CrossRef]
- Kerridge, A.; Lappin-Scott, H.; Stevens, J.R. Antibacterial properties of larval secretions of the blowfly, Lucilia sericata. Med. Vet. Entomol. 2005, 19, 333–337. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhang, B.; Liu, H.; Song, W.; He, J.; Lv, D.; Wang, S.; Xu, X. Activity of antimicrobial protein from maggots against Staphylococcus aureus in vitro and in vivo. Int. J. Mol. Med. 2013, 31, 1159–1165. [Google Scholar]
- Kruglikova, A.A.; Chernysh, S.I. Antimicrobial compounds from the excretions of surgical maggots, Lucilia sericata (Meigen) (Diptera, Calliphoridae). Entomol. Rev. 2011, 91, 813–819. [Google Scholar] [CrossRef]
- Čeřovský, V.; Slaninová, J.; Fučík, V.; Monincová, L.; Bednárová, L.; Maloň, P.; Štokrová, J. Lucifensin, a novel insect defensin of medicinal maggots: Synthesis and structural study. ChemBioChem 2011, 12, 1352–1361. [Google Scholar] [CrossRef]
- Nygaard, M.K.E.; Andersen, A.S.; Kristensen, H-H.; Krogfelt, K.A.; Fojan, P.; Wimmer, R. The insect defensin lucifensin from Lucilia sericata. J. Biomol. NMR 2012, 52, 277–282. [Google Scholar] [CrossRef]
- Takeuchi, K.; Takahashi, H.; Sugai, M.; Iwai, H.; Kohno, T.; Sekimizu, K.; Natori, S.; Shimada, I. Channel-forming membrane permeabilization by an antimicrobial protein, sapecin. J. Biol. Chem. 2004, 279, 4981–4987. [Google Scholar]
- Pymol. Available online: http://www.pymol.org/ (accessed on 12 February 2014).
- Cociancich, S.; Bulet, P.; Hetru, C.; Hoffmann, J.A. The inducible antimicrobial peptides of insects. Parasitol. Today 1994, 10, 132–138. [Google Scholar] [CrossRef]
- Altincicek, B.; Vilcinskas, A. Septic injury-inducible genes in medicinal maggots of the green blow fly Lucilia sericata. Insect Mol. Biol. 2009, 18, 119–125. [Google Scholar] [CrossRef]
- Andersen, A.S.; Sandvang, D.; Schnorr, K.M.; Kruse, T.; Neve, S.; Joergensen, B.; Karlsmark, T.; Krogfelt, K.A. A novel approach to the antimicrobial activity of maggot debridement therapy. J. Antimicrob. Chemother. 2010, 65, 1646–1654. [Google Scholar] [CrossRef]
- Valachová, I.; Bohová, J.; Pálošová, Z.; Takáč, P.; Kozánek, M.; Majtán, J. Expression of lucifensin in Lucilia sericata medicinal maggots in infected environments. Cell Tissue Res. 2013, 353, 165–171. [Google Scholar] [CrossRef]
- Bém, R.; Jirkovská, A.; Fejfarová, V.; Dubský, M.; Skibová, J.; Čeřovský, V. Acute antimicrobial effect of maggot therapy on diabetic foot ulcer infection as a basis for identification of antimicrobial peptides from maggots (Abstract). Diabetologia 2010, 53, 56. [Google Scholar]
- Bowling, F.L.; Salgami, E.V.; Boulton, A.J. Larval therapy: a novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers. Diabetes Care 2007, 30, 370–371. [Google Scholar] [CrossRef]
- Harder, J.; Meyer-Hoffert, U.; Wehkamp, K.; Schwichtenberg, L.; Schroder, J.M. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J. Invest. Dermatol. 2004, 123, 522–529. [Google Scholar] [CrossRef]
- Khanolkar, M.P.; Bain, S.C.; Stephens, J.W. The diabetic foot. QJM 2008, 101, 685–695. [Google Scholar] [CrossRef]
- Lobmann, R.; Schultz, G.; Lehnert, H. Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care 2005, 28, 461–471. [Google Scholar] [CrossRef]
- Rivas-Santiago, B.; Trujillo, V.; Montoya, A.; Gonzalez-Curiel, I.; Castaneda-Delgado, J.; Cardenas, A.; Rincon, K.; Hernandez, M.L.; Hernandez-Pando, R. Expression of antimicrobial peptides in diabetic foot ulcer. J. Dermatol. Sci. 2012, 65, 19–26. [Google Scholar] [CrossRef]
- Van der Plas, M.J.A; van der Does, A.M; Baldry, M.; Dogterom-Ballering, H.C.M; van Gulpen, C; van Dissel, J.T.; Nibbering, P.H; Jukema, G.N. Maggot excretions/secretions inhibit multiple neutrophil pro-inflammatory responses. Microbes Infect. 2007, 9, 507–514. [Google Scholar] [CrossRef]
- Horobin, A.J.; Shakesheff, K.M.; Pritchard, D.I. Promotion of human dermal fibroblast migration, matrix remodeling and modification of fibroblast morphology within a novel 3D model by Lucilia sericata larval secretions. J. Invest. Dermatol. 2006, 126, 1410–1418. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Čeřovský, V.; Bém, R. Lucifensins, the Insect Defensins of Biomedical Importance: The Story behind Maggot Therapy. Pharmaceuticals 2014, 7, 251-264. https://doi.org/10.3390/ph7030251
Čeřovský V, Bém R. Lucifensins, the Insect Defensins of Biomedical Importance: The Story behind Maggot Therapy. Pharmaceuticals. 2014; 7(3):251-264. https://doi.org/10.3390/ph7030251
Chicago/Turabian StyleČeřovský, Václav, and Robert Bém. 2014. "Lucifensins, the Insect Defensins of Biomedical Importance: The Story behind Maggot Therapy" Pharmaceuticals 7, no. 3: 251-264. https://doi.org/10.3390/ph7030251




