Radiolabeled Cetuximab Conjugates for EGFR Targeted Cancer Diagnostics and Therapy †
Abstract
:1. Introduction
2. Cetuximab Combined with Radiotherapy
3. Radiolabeled Cetuximab
| Radionuclide | Half-life | Main types of decay (probability) b | Emax (MeV) | Production |
|---|---|---|---|---|
| Radionuclides for imaging | ||||
| 64Cu | 12.7 h | β+ (17.5%) | 0.653 | cyclotron |
| β− (38.5%) | 0.579 | 64Ni(p,n)64Cu | ||
| EC (43.5%) | 1.675 | |||
| 68Ga | 1.13 h | β+ (87.7%) | 1.899 | 68Ge/68Ga generator |
| EC (8.9%) | 2.921 | |||
| γ (3.2%) | 1.077 | |||
| 86Yc | 14.7 h | β+ (11.9/5.6%) | 1.221/1.545 | cyclotron |
| γ (83/32.6%) | 1.077/0.628 | 86Sr(p,n)86Y | ||
| 89Zrc | 3.3 dβ+ (22.7%) | 0.902 | cyclotron | |
| γ(100%) | 0.909 | 89Y(p,n)89Zr | ||
| 99mTc | 6 h | γ (99%) | 0.141 | 99Mo/99mTc generator |
| 111In | 2.8 d | γ (100%) | 0.245 | cyclotron |
| EC (99.99%) | 0.417 | 111Cd(p,n)111In | ||
| 124Ic | 4.2 d | β+ (11.7/10.8%) | 1.535/2.135 | cyclotron |
| γ (63/10.9%) | 0.603/1.691 | 124Te(p,n)124I | ||
| 125I | 59.4 d | γ (100%) | 0.035 | nuclear reactor |
| EC (100%) | 0.150 | 124Xe(n,γ)125Xe→125I | ||
| 90Y | 2.67 d | β− (99.98%) | 2.279 | 90Sr/90Y generator |
| 131I | 8 d | β− (89.4/7.4%) | 0.606/0.334 | nuclear reactor |
| γ (83.1/7.3%) | 0.364/0.637 | 130Te(n,γ)131Te→131I | ||
| 177Lu | 6.65 d | β− (79.3/11.6%) | 0.498/0.177 | nuclear reactor |
| γ (20.3/11%) | 0.113/0.208 | 176Yb(n,γ)177Yb→177Lu | ||
| 213Bi | 45.6 min | α (1.9%) | 5.981 | 225Ac/213Bi generator |
| β− (66.2/30.8%) | 1.423/0.983 | |||
3.1. Radionuclides
3.1.1. Radionuclides for C225 Conjugates Used as Imaging Probes
3.1.2. Radionuclides for Cetuximab Conjugates Used as Therapeutics
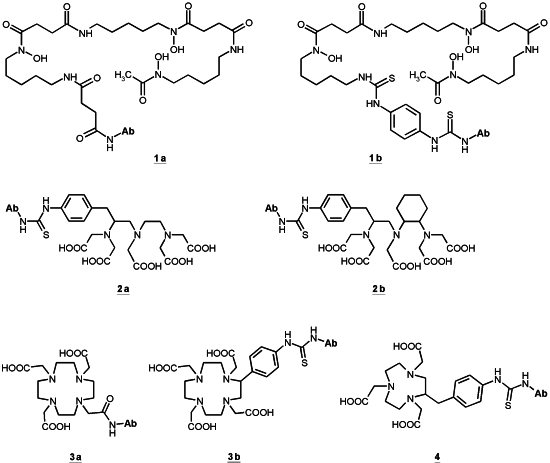
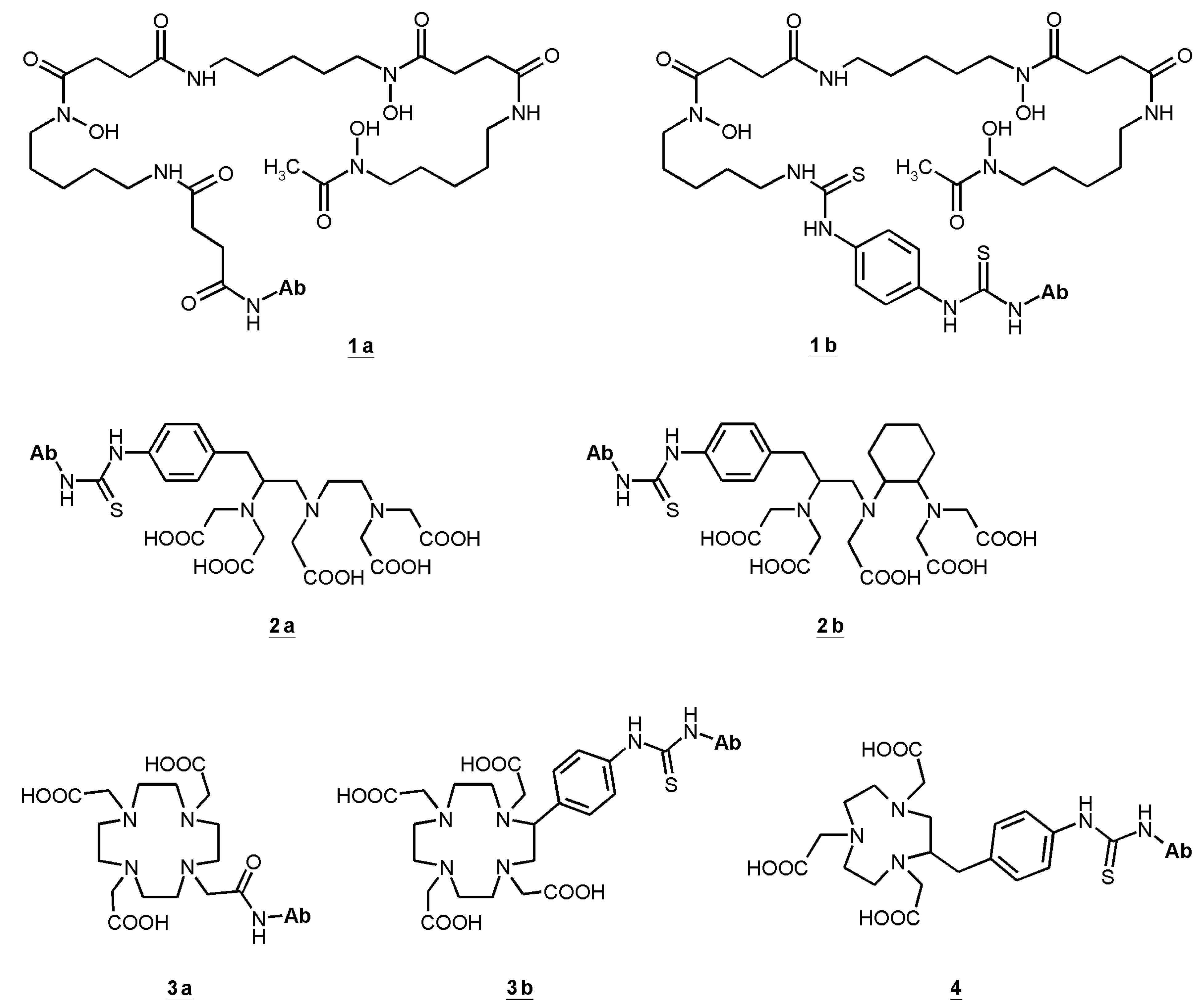
3.2. Linking Chelating Units
| Radionuclide | Chelator | Tumor type | Application | Tumor uptake | Tumor/muscle ratio | Liver uptake | Reference |
|---|---|---|---|---|---|---|---|
| (%ID/g, 24 h post-injection) | |||||||
| 64Cu | DOTA | h GB | i.v. | 12.5 | 5 | 15 | [59] |
| h PC | 11 | 4.5 | 6 (rat) | ||||
| h CRC | ~5 | 2 | |||||
| m CRC | 10 | 4 | |||||
| h M | |||||||
| 64Cu | DOTA | h CC | i.v. | 14 | 3.5 | 16 | [60] |
| 64Cu | DOTA | PC-3 | i.v. | 15 | 15 | 17 | [151] |
| 64Cu | DOTA | A431 | i.v. | 18.5 | 8.5 | 13 | [61] |
| h M | 2.6 | 1.3 | 10 | ||||
| 64Cu | DOTA | h HNSCC (UMSCC22B) | i.v. | 19 | 6 | 11 | [117] a |
| 64Cu | DOTA | h HNSCC (UMSCC1) | i.v. | 6 | 2.5 | 13 | [117] a |
| 64Cu | NOTA | m BC | i.v. | 4 | 4 | 19 | [155] |
| 64Cu | NOTA | m BC | i.v. | 20 | 10 | 19 | [54] |
| 66Ga | NOTA | h BC | i.v. | 4 | 5 | 6 | [72] b |
| 86Y | DTPA | h CRC | i.v. | 21 | 11 | 10 | [83] |
| 88Y | DTPA | A431 | i.p. | 21 | 14 | 11 | [74] |
| 88Y | DOTA | A431 | i.p. | 17 | 11 | 10 | [74] |
| 89Zr | Df | h GB | i.v. | 15 | 15 | 10-12 | [141] |
| h CRC | 10 | 10 | |||||
| A431 | 8 | 8 | |||||
| h BC | 3 | 3 | |||||
| 89Zr | Df | A431 | i.v. | 3.5c | 10d | 11c | [142] |
| 89Zr | Df | A431 | i.p. | 21 | 17 | 10 | [74] |
| 89Zr | Df | A431 | i.v. | 15 | 8 | 9 | [139] |
| 89Zr + | Df | A431 | i.v. | 22 | 19 | 20 | [156] |
| 89Zr + ½ dye e | Df | 20 | 19 | 22 | |||
| 89Zr + 1 dye | Df | 20 | 19 | 25 | |||
| 89Zr + 2 dye | Df | 13 | 16 | 40 | |||
| 99mTc | EC | h BC | i.v. | 0.3 | 8.5 | 0.6 | [91] |
| 86Y | DTPA | h CRC | i.v. | 21 | 11 | 10 | [83] |
| 88Y | DTPA | A431 | i.p. | 21 | 14 | 11 | [74] |
| 88Y | DOTA | A431 | i.p. | 17 | 11 | 10 | [74] |
| 90Y | DOTA | normal rats | i.v. | 2 | [157] | ||
| 177Lu | DOTA | A431 | i.p. | 18 | 12 | 13 | [109] |
| 177Lu | DOTA | A431 | i.p. | 17.5 | 12 | 8-13 | [74] |
| 177Lu | DTPA | A431 | i.p. | 17.5 | 12 | 7 | [74] |
| 111In | DTPA | A431 | i.v. | 11 | 29 | 47 | [158] f |
| DTPA-PEG | A431 | 8.7 | 13 | 25 | |||
| 111In | DTPA | h OC | i.v. | 8.8 | 11 | 4 | [95] f |
| 111In | DTPA | h CRC | i.v. | 28/24g | 28/24g | 9/16g | [85] |
| h PC | 16 | 16 | 6 | ||||
| h PancC | 10 | 10 | 10 | ||||
| h OC | 13 | 13 | 10 | ||||
| h M | 3 | 3 | 9 | ||||
| 111In | DTPA | h HNSCC | i.v. | 20 | 14 | 11 | [108] |
| 111In | DTPA | h BC | i.v. | 18/40f | 13 | 11/15f | [135] |
| 111In | DTPA | h HNSCC (FaDu) | i.v. | 27 | 13 | 8 | [159] |
| 125I | h HNSCC | i.v. | 11 | 8 | 7 | [108] | |
| 125I | A431 | i.p. | 8.4 | 5.6 | 4 | [109] | |
| 125I | A431 | i.p. | 8 | 5 | 4 | [74] | |
3.3. Liver Accumulation
3.4. The Enhanced Permeability and Retention Effect
3.5. Therapeutic Approaches with Labeled Cetuximab
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gunderson, L.L.; Ashman, J.B.; Haddock, M.G.; Petersen, I.A.; Moss, A.; Heppell, J.; Gray, R.J.; Pockaj, B.A.; Nelson, H.; Beauchamp, C. Integration of radiation oncology with surgery as combined-modality treatment. Surg. Oncol. Clin. N. Am. 2013, 22, 405–432. [Google Scholar] [CrossRef]
- Galaal, K.; van der Heijden, E.; Godfrey, K.; Naik, R.; Kucukmetin, A.; Bryant, A.; Das, N.; Lopes, A.D. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma. Cochrane Database Syst. Rev. 2013, 2, CD006812. [Google Scholar]
- Yang, H.; Diao, L.Q.; Shi, M.; Ma, R.; Wang, J.H.; Li, J.P.; Xiao, F.; Xue, Y.; Xu, M.; Zhou, B. Efficacy of intensity-modulated radiotherapy combined with chemotherapy or surgery in locally advanced squamous cell carcinoma of the head-and-neck. Biologics 2013, 7, 223–229. [Google Scholar]
- Pignon, J.P.; le Maître, A.; Maillard, E.; Bourhis, J.; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Bourhis, J.; Overgaard, J.; Audry, H.; Ang, K.K.; Saunders, M.; Bernier, J.; Horiot, J.C.; le Maître, A.; Pajak, T.F.; Poulsen, M.G.; et al. Meta-Analysis of Radiotherapy in Carcinomas of Head and neck (MARCH) Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006, 368, 843–854. [Google Scholar] [CrossRef]
- Marquardt, H.; Hunkapiller, M.W.; Hood, L.E.; Twardzik, D.R.; De Larco, J.E.; Stephenson, J.R.; Todaro, G.J. Transforming growth factors produced by retrovirus-transformed rodent fibroblasts and human melanoma cells: Amino acid sequence homology with epidermal growth factor. Proc. Natl. Acad. Sci. USA 1983, 80, 4684–4688. [Google Scholar] [CrossRef]
- Higashiyama, S.; Abraham, J.A.; Miller, J.; Fiddes, J.C.; Klagsbrun, M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 1991, 251, 936–939. [Google Scholar] [CrossRef]
- Ciardiello, F.; Kim, N.; Saeki, T.; Dono, R.; Persico, M.G.; Plowman, G.D.; Garrigues, J.; Radke, S.; Todaro, G.J.; Salomon, D.S. Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc. Natl. Acad. Sci. USA 1991, 88, 7792–7796. [Google Scholar] [CrossRef]
- Sasada, R.; Ono, Y.; Taniyama, Y.; Shing, Y.; Folkman, J.; Igarashi, K. Cloning and expression of cDNA encoding human betacellulin, a new member of the EGF family. Biochem. Biophys. Res. Commun. 1993, 190, 1173–1179. [Google Scholar] [CrossRef]
- Olayioye, M.A.; Neve, R.M.; Lane, H.A.; Hynes, N.E. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000, 19, 3159–3167. [Google Scholar] [CrossRef]
- Alroy, I.; Yarden, Y. The ErbB signaling network in embryogenesis and oncogenesis: Signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997, 410, 83–86. [Google Scholar] [CrossRef]
- Lewis, T.S.; Shapiro, P.S.; Ahn, N.G. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 1998, 74, 49–139. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef]
- Silva, C.M. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene 2004, 23, 8017–8023. [Google Scholar] [CrossRef]
- Pensa, S.; Regis, G.; Boselli, D.; Novelli, G.; Poli, V. STAT1 and STAT3 in Tumorigenesis: Two sides of the same coin? Madame Curie Bioscience Database. 2009. Chapter 8. pp. 100–121. Available online: http://www.ncbi.nlm.nih.gov/books/NBK6568/ (accessed on 27 February 2014).
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 1995, 19, 183–232. [Google Scholar] [CrossRef]
- Humblet, Y. Cetuximab: An IgG(1) monoclonal antibody for the treatment of epidermal growth factor receptor-expressing tumours. Expert Opin. Pharmacother. 2004, 5, 1621–1633. [Google Scholar] [CrossRef]
- Harding, J.; Burtness, B. Cetuximab: An epidermal growth factor receptor chemeric human-murine monoclonal antibody. Drugs Today 2005, 41, 107–127. [Google Scholar] [CrossRef]
- Santiago, A.; Eicheler, W.; Bussink, J.; Rijken, P.; Yaromina, A.; Beuthien-Baumann, B.; van der Kogel, A.J.; Baumann, M.; Krause, M. Effect of cetuximab and fractionated irradiation on tumour micro-environment. Radiother. Oncol. 2010, 97, 322–329. [Google Scholar] [CrossRef]
- Naramura, M.; Gillies, S.D.; Mendelsohn, J.; Reisfeld, R.A.; Mueller, B.M. Therapeutic potential of chimeric and murine anti-(epidermal growth factor receptor) antibodies in a metastasis model for human melanoma. Cancer Immunol. Immunother. 1993, 37, 343–349. [Google Scholar] [CrossRef]
- Goldstein, N.I.; Prewett, M.; Zuklys, K.; Rockwell, P.; Mendelsohn, J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin. Cancer Res. 1995, 1, 1311–1318. [Google Scholar]
- De Bono, J.S.; Rowinsky, E.K. The ErbB receptor family: A therapeutic target for cancer. Trends Mol. Med. 2002, 8, S19–S26. [Google Scholar] [CrossRef]
- Wu, X.; Rubin, M.; Fan, Z.; DeBlasio, T.; Soos, T.; Koff, A.; Mendelsohn, J. Involvement of p27KIP1 in G1 arrest mediated by an anti-epidermal growth factor receptor monoclonal antibody. Oncogene 1996, 12, 1397–1403. [Google Scholar]
- Peng, D.; Fan, Z.; Lu, Y.; De Blasio, T.; Scher, H.; Mendelsohn, J. Anti-epidermal growth factor receptor monoclonal antibody 225 up-regulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996, 56, 3666–3669. [Google Scholar]
- Huang, S.M.; Bock, J.M.; Harari, P.M. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999, 59, 1935–1940. [Google Scholar]
- Baumann, M.; Krause, M.; Dikomey, E.; Dittmann, K.; Dörr, W.; Kasten-Pisula, U.; Rodemann, H.P. EGFR-targeted anti-cancer drugs in radiotherapy: Preclinical evaluation of mechanisms. Radiother. Oncol. 2007, 83, 238–248. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef]
- Pan, Q.; Gorin, M.A.; Teknos, T.N. Pharmacotherapy of head and neck squamous cell carcinoma. Expert. Opin. Pharmacother. 2009, 10, 2291–302. [Google Scholar] [CrossRef]
- Socinski, M.A.; Evans, T.; Gettinger, S.; Hensing, T.A.; Sequist, L.V.; Ireland, B.; Stinchcombe, T.E. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed; American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e341S–e368S. [Google Scholar] [CrossRef]
- Faloppi, L.; Andrikou, K.; Cascinu, S. Cetuximab: Still an option in the treatment of pancreatic cancer? Expert Opin. Biol. Ther. 2013, 13, 791–801. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Bernier, J.; Schneider, D. Cetuximab combined with radiotherapy: An alternative to chemoradiotherapy for patients with locally advanced squamous cell carcinomas of the head and neck? Eur. J. Cancer 2007, 43, 35–45. [Google Scholar] [CrossRef]
- Caudell, J.J.; Sawrie, S.M.; Spencer, S.A.; Desmond, R.A.; Carroll, W.R.; Peters, G.E.; Nabell, L.M.; Meredith, R.F.; Bonner, J.A. Locoregionally advanced head and neck cancer treated with primary radiotherapy: A comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 676–681. [Google Scholar] [CrossRef]
- Agulnik, M. New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN). Med. Oncol. 2012, 29, 2481–2491. [Google Scholar] [CrossRef]
- Robert, F.; Ezekiel, M.P.; Spencer, S.A.; Meredith, R.F.; Bonner, J.A.; Khazaeli, M.B.; Saleh, M.N.; Carey, D.; LoBuglio, A.F.; Wheeler, R.H.; et al. Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J. Clin. Oncol. 2001, 19, 3234–3243. [Google Scholar]
- Dattatreya, S.; Goswami, C. Cetuximab plus radiotherapy in patients with unresectable locally advanced squamous cell carcinoma of head and neck region—A open labelled single arm phase II study. Indian J. Cancer 2011, 48, 154–157. [Google Scholar] [CrossRef]
- Ang, K.K.; Zhang, Q.E.; Rosenthal, D.I.; Nguyen-Tan, P.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Schwartz, D.L.; El-Naggar, A.K.; Gillison, M.L.; et al. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC). J. Clin. Oncol. 2011, 29, 5500. [Google Scholar]
- Eriksen, J.G.; Maare, C.; Johansen, J.; Primdahl, H.; Evensen, J.; Kristensen, C.A.; Andersen, L.J.; Overgaard, J. A randomized phase III study of primary curative (chemo)-radiotherapy and the egfr-inhibitor zalutumumab for squamous cell carcinoma of the head and neck (HNSCC). ESMO 2013, 12, 5–6. [Google Scholar]
- Walsh, L.; Gillham, C.; Dunne, M.; Fraser, I.; Hollywood, D.; Armstrong, J.; Thirion, P. Toxicity of cetuximab versus cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell cancer (LAHNSCC). Radiother. Oncol. 2011, 98, 38–41. [Google Scholar] [CrossRef]
- Gurtner, K.; Deuse, Y.; Bütof, R.; Schaal, K.; Eicheler, W.; Oertel, R.; Grenman, R.; Thames, H.; Yaromina, A.; Baumann, M.; et al. Diverse effects of combined radiotherapy and EGFR inhibition with antibodies or TK inhibitors on local tumour control and correlation with EGFR gene expression. Radiother. Oncol. 2011, 99, 323–330. [Google Scholar] [CrossRef]
- Stegeman, H.; Kaanders, J.H.; van der Kogel, A.J.; Iida, M.; Wheeler, D.L.; Span, P.N.; Bussink, J. Predictive value of hypoxia, proliferation and tyrosine kinase receptors for EGFR-inhibition and radiotherapy sensitivity in head and neck cancer models. Radiother. Oncol. 2013, 106, 383–389. [Google Scholar] [CrossRef]
- Sok, J.C.; Coppelli, F.M.; Thomas, S.M.; Lango, M.N.; Xi, S.; Hunt, J.L.; Freilino, M.L.; Graner, M.W.; Wikstrand, C.J.; Bigner, D.D.; et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin. Cancer Res. 2006, 12, 5064–5073. [Google Scholar] [CrossRef]
- Chen, L.F.; Cohen, E.E.; Grandis, J.R. New strategies in head and neck cancer: Understanding resistance to epidermal growth factor receptor inhibitors. Clin. Cancer Res. 2010, 16, 2489–2495. [Google Scholar] [CrossRef]
- Hubbard, J.M.; Alberts, S.R. Alternate dosing of cetuximab for patients with metastatic colorectal cancer. Gastrointest. Cancer Res. 2013, 6, 47–55. [Google Scholar]
- Smilek, P.; Neuwirthova, J.; Jarkovsky, J.; Dusek, L.; Rottenberg, J.; Kostrica, R.; Srovnal, J.; Hajduch, M.; Drabek, J.; Klozar, J. Epidermal growth factor receptor (EGFR) expression and mutations in the EGFR signaling pathway in correlation with anti-EGFR therapy in head and neck squamous cell carcinomas. Neoplasma 2012, 59, 508–515. [Google Scholar] [CrossRef]
- Bardelli, A.; Jänne, P.A. The road to resistance: EGFR mutation and cetuximab. Nat. Med. 2012, 18, 199–200. [Google Scholar] [CrossRef]
- Corcoran, E.B.; Hanson, R.N. Imaging EGFR and HER2 by PET and SPECT: A Review. Med. Res. Rev. 2013. [Google Scholar] [CrossRef]
- LNHB. Available online: http://www.nucleide.org/DDEP_WG/DDEPdata.htm (accessed on 25 February 2014).
- Lubberink, M.; Herzog, H. Quantitative imaging of 124I and 86Y with PET. Eur. J. Nucl. Med. Mol. Imaging. 2011, 38, S10–S18. [Google Scholar] [CrossRef]
- FDA Data Specification. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125084s 168lbl.pdf (accessed on 25 February 2014).
- Milenic, D.E.; Brady, E.D.; Brechbiel, M.W. Antibody-targeted radiation cancer therapy. Nat. Rev. Drug Discov. 2004, 3, 488–499. [Google Scholar] [CrossRef]
- Srivastava, S.; Dadachova, E. Recent advances in radionuclide therapy. Semin. Nucl. Med. 2001, 31, 330–341. [Google Scholar] [CrossRef]
- Guo, Y.; Parry, J.J.; Laforest, R.; Rogers, B.E.; Anderson, C.J. The role of p53 in combination radioimmunotherapy with 64Cu-DOTA-cetuximab and cisplatin in a mouse model of colorectal cancer. J. Nucl. Med. 2013, 54, 1621–1629. [Google Scholar] [CrossRef]
- Szelecsenyi, F.; Blessing, G.; Qaim, S.M. Excitation function of proton induced nuclear reactions on enriched 61Ni and 64Ni: Possibility of production of no-carrier-added 61Cu and 64Cu at a small cyclotron. Appl. Radiat. Isot. 1993, 44, 575–580. [Google Scholar] [CrossRef]
- McCarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997, 24, 35–2443. [Google Scholar] [CrossRef]
- Linder, M.C.; Hazegh-Azam, M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar]
- Anderson, C.J.; Ferdani, R. Copper-64 radiopharmaceuticals for PET imaging of cancer: Advances in preclinical and clinical research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef]
- Cai, W.; Chen, K.; He, L.; Cao, Q.; Koong, A.; Chen, X. Quantitative PET of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 850–858. [Google Scholar] [CrossRef]
- Eiblmaier, M.; Meyer, L.A.; Watson, M.A.; Fracasso, P.M.; Pike, L.J.; Anderson, C.J. Correlating EGFR expression with receptor-binding properties and internalization of 64Cu-DOTA-cetuximab in 5 cervical cancer cell lines. J. Nucl. Med. 2008, 49, 1472–1479. [Google Scholar] [CrossRef]
- Ping Li, W.; Meyer, L.A.; Capretto, D.A.; Sherman, C.D.; Anderson, C.J. Receptor-binding, biodistribution, and metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biother. Radiopharm. 2008, 23, 158–171. [Google Scholar] [CrossRef]
- Niu, G.; Li, Z.; Xie, J.; Le, Q.T.; Chen, X. PET of EGFR antibody distribution in head and neck squamous cell carcinoma models. J. Nucl. Med. 2009, 50, 1116–1123. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Engle, J.W.; Bean, J.; Yang, Y.; Leigh, B.R.; Barnhart, T.E.; Cai, W. Positron emission tomography imaging of CD105 expression with a 64Cu-labeled monoclonal antibody: NOTA is superior to DOTA. PLoS One 2011, 6, e28005. [Google Scholar]
- Velikyan, I.; Sundberg, A.L.; Lindhe, O.; Höglund, A.U.; Eriksson, O.; Werner, E.; Carlsson, J.; Bergström, M.; Långström, B.; Tolmachev, V. Preparation and evaluation of 68Ga-DOTA-hEGF for visualization of EGFR expression in malignant tumors. J. Nucl. Med. 2005, 46, 1881–1888. [Google Scholar]
- Liu, Z.; Cui, L.; Liu, X.; Wang, F. Noninvasive small-animal PET of trastuzumab-mediated EGFR down-regulation with 68Ga-Vec(Fab’)2. J. Nucl. Med. 2012, 53, 342. [Google Scholar]
- Strand, J.; Honarvar, H.; Perols, A.; Orlova, A.; Selvaraju, R.K.; Karlström, A.E.; Tolmachev, V. Influence of macrocyclic chelators on the targeting properties of 68Ga-labeled synthetic affibody molecules: Comparison with 111In-labeled counterparts. PLoS One 2013, 8, e70028. [Google Scholar]
- Vosjan, M.J.; Perk, L.R.; Roovers, R.C.; Visser, G.W.; Stigter-van Walsum, M.; van Bergen, E.; Henegouwen, P.M.; van Dongen, G.A. Facile labelling of an anti-epidermal growth factor receptor Nanobody with 68Ga via a novel bifunctional desferal chelate for immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 753–763. [Google Scholar] [CrossRef]
- Griffiths, G.L.; Chang, C.H.; McBride, W.J.; Rossi, E.A.; Sheerin, A.; Tejada, G.R.; Karacay, H.; Sharkey, R.M.; Horak, I.D.; Hansen, H.J.; et al. Reagents and methods for PET using bispecific antibody pretargeting and 68Ga-radiolabeled bivalent hapten-peptide-chelate conjugates. J. Nucl. Med. 2004, 45, 30–39. [Google Scholar]
- Schuhmacher, J.; Klivényi, G.; Kaul, S.; Henze, M.; Matys, R.; Hauser, H.; Clorius, J. Pretargeting of human mammary carcinoma xenografts with bispecific anti-MUC1/anti-Ga chelate antibodies and immunoscintigraphy with PET. Nucl. Med. Biol. 2001, 28, 821–828. [Google Scholar] [CrossRef]
- Kuijpers, W.H.; Bos, E.S.; Kaspersen, F.M.; Veeneman, G.H.; van Boeckel, C.A. Specific recognition of antibody-oligonucleotide conjugates by radiolabeled antisense nucleotides: A novel approach for two-step radioimmunotherapy of cancer. Bioconjug. Chem. 1993, 4, 94–102. [Google Scholar] [CrossRef]
- Rusckowski, M.; Qu, T.; Chang, F.; Hnatowich, D.J. Pretargeting using peptide nucleic acid. Cancer 1997, 80, 2699–2705. [Google Scholar] [CrossRef]
- Engle, J.W.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Myklejord, D.V.; Barnhart, T.E.; Theuer, C.P.; Nickles, R.J.; Cai, W. Positron Emission Tomography Imaging of Tumor Angiogenesis with a 66Ga-Labeled Monoclonal Antibody. Mol. Pharm. 2012, 9, 1441–1448. [Google Scholar] [CrossRef]
- Garmestani, K.; Milenic, D.E.; Plascjak, P.S.; Brechbiel, M.W. A new and convenient method for purification of 86Y using a Sr(II) selective resin and comparison of biodistribution of 86Y and 111In labeled Herceptin. Nucl. Med. Biol. 2002, 29, 599–606. [Google Scholar] [CrossRef]
- Perk, L.R.; Visser, G.W.; Vosjan, M.J.; Stigter-van Walsum, M.; Tijink, B.M.; Leemans, C.R.; van Dongen, G.A. 89Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals 90Y and 177Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J. Nucl. Med. 2005, 46, 1898–1906. [Google Scholar]
- Pentlow, K.S.; Finn, R.D.; Larson, S.M.; Erdi, Y.E.; Beattie, B.J.; Humm, J.L. Quantitative Imaging of Yttrium-86 with PET. The Occurrence and Correction of Anomalous Apparent Activity in High Density Regions. Clin. Positron Imaging 2000, 3, 85–90. [Google Scholar] [CrossRef]
- Walrand, S.; Jamar, F.; Mathieu, I.; de camps, J.; Lonneux, M.; Sibomana, M.; Labar, D.; Michel, C.; Pauwels, S. Quantitation in PET using isotopes emitting prompt single gammas: Application to yttrium-86. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 354–361. [Google Scholar] [CrossRef]
- Nayak, T.K.; Brechbiel, M.W. Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjug Chem. 2009, 20, 825–841. [Google Scholar] [CrossRef]
- Lövqvist, A.; Humm, J.L.; Sheikh, A.; Finn, R.D.; Koziorowski, J.; Ruan, S.; Pentlow, K.S.; Jungbluth, A.; Welt, S.; Lee, F.T.; et al. PET imaging of 86Y-labe.led anti-Lewis Y monoclonal antibodies in a nude mouse model: Comparison between 86Y and (111)In radiolabels. J. Nucl. Med. 2001, 42, 1281–1287. [Google Scholar]
- Palm, S.; Enmon, R.M., Jr.; Matei, C.; Kolbert, K.S.; Xu, S.; Zanzonico, P.B.; Finn, R.L.; Koutcher, J.A.; Larson, S.M.; Sgouros, G. Pharmacokinetics and Biodistribution of 86Y-Trastuzumab for 90Y dosimetry in an ovarian carcinoma model: Correlative MicroPET and MRI. J. Nucl. Med. 2003, 44, 1148–1155. [Google Scholar]
- Schneider, D.W.; Heitner, T.; Alicke, B.; Light, D.R.; McLean, K.; Satozawa, N.; Parry, G.; Yoo, J.; Lewis, J.S.; Parry, R. In vivo biodistribution, PET imaging, and tumor accumulation of 86Y- and 111In-antimindin/RG-1, engineered antibody fragments in LNCaP tumor-bearing nude mice. J. Nucl. Med. 2009, 50, 435–443. [Google Scholar]
- Nayak, T.K.; Garmestani, K.; Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. Preparation, biological evaluation, and pharmacokinetics of the human anti-HER1 monoclonal antibody panitumumab labeled with 86Y for quantitative PET of carcinoma. J. Nucl. Med. 2010, 51, 942–950. [Google Scholar] [CrossRef]
- Wong, K.J.; Baidoo, K.E.; Nayak, T.K.; Garmestani, K.; Brechbiel, M.W.; Milenic, D.E. In Vitro and In Vivo Pre-Clinical Analysis of a F(ab')(2) Fragment of Panitumumab for Molecular Imaging and Therapy of HER1 Positive Cancers. EJNMMI Res. 2011, 1, 1. [Google Scholar] [CrossRef]
- Nayak, T.K.; Regino, C.A.; Wong, K.J.; Milenic, D.E.; Garmestani, K.; Baidoo, K.E.; Szajek, L.P.; Brechbiel, M.W. PET imaging of HER1-expressing xenografts in mice with 86Y-CHX-A′′-DTPA-cetuximab. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1368–1376. [Google Scholar] [CrossRef]
- Nayak, T.K.; Garmestani, K.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. HER1-targeted 86Y-panitumumab possesses superior targeting characteristics than 86Y-cetuximab for PET imaging of human malignant mesothelioma tumors xenografts. PLoS One 2011, 6, e18198. [Google Scholar]
- Milenic, D.E.; Wong, K.J.; Baidoo, K.E.; Ray, G.L.; Garmestani, K.; Williams, M.; Brechbiel, M.W. Cetuximab: Preclinical evaluation of a monoclonal antibody targeting EGFR for radioimmunodiagnostic and radioimmunotherapeutic applications. Cancer Biother Radiopharm. 2008, 23, 619–631. [Google Scholar] [CrossRef]
- Verel, I.; Visser, G.W.; Boellaard, R.; Stigter-van Walsum, M.; Snow, G.B.; van Dongen, G.A. 89Zr immuno-PET: Comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 2003, 44, 1271–1281. [Google Scholar]
- FDA-Specification. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125084s225lbl.pdf (accessed on 25 February 2014).
- Börjesson, P.K.; Jauw, Y.W.; de Bree, R.; Roos, J.C.; Castelijns, J.A.; Leemans, C.R.; van Dongen, G.A.; Boellaard, R. Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J. Nucl. Med. 2009, 50, 1828–1836. [Google Scholar] [CrossRef]
- Perk, L.R.; Visser, O.J.; Stigter-van Walsum, M.; Vosjan, M.J.; Visser, G.W.; Zijlstra, J.M.; Huijgens, P.C.; van Dongen, G.A. Preparation and evaluation of 89Zr-Zevalin for monitoring of 90Y-Zevalin biodistribution with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1337–1345. [Google Scholar] [CrossRef]
- Van Nerom, C.G.; Bormans, G.M.; de Roo, M.J.; Verbruggen, A.M. First experience in healthy volunteers with technetium-99m L,L-ethylenedicysteine, a new renal imaging agent. Eur. J. Nucl. Med. 1993, 20, 738–746. [Google Scholar]
- Schechter, N.R.; Yang, D.J.; Azhdarinia, A.; Kohanim, S.; Wendt, R.; Oh, C.S.; Hu, M.; Yu, D.F.; Bryant, J.; Ang, K.K.; et al. Assessment of epidermal growth factor receptor with 99mTc-ethylenedicysteine-C225 monoclonal antibody. Anticancer Drugs 2003, 14, 49–56. [Google Scholar] [CrossRef]
- Schechter, N.R.; Wendt, R.E.; Yang, D.J.; Azhdarinia, A.; Erwin, W.D.; Stachowiak, A.M.; Broemeling, L.D.; Kim, E.E.; Cox, J.D.; Podoloff, D.A.; et al. Radiation dosimetry of 99mTc-labeled C225 in patients with squamous cell carcinoma of the head and neck. J. Nucl. Med. 2004, 45, 1683–1687. [Google Scholar]
- Kaur, S.; Venktaraman, G.; Jain, M.; Senapati, S.; Garg, P.K.; Batra, S.K. Recent trends in antibody-based oncologic imaging. Cancer Lett. 2012, 31, 97–111. [Google Scholar]
- Capello, A.; Krenning, E.P.; Breeman, W.A.; Bernard, B.F.; de Jong, M. Peptide receptor radionuclide therapy in vitro using [111In-DTPA0]octreotide. J. Nucl. Med. 2003, 44, 98–104. [Google Scholar]
- Huhtala, T.; Laakkonen, P.; Sallinen, H.; Ylä-Herttuala, S.; Närvänen, A. In vivo SPECT/CT imaging of human orthotopic ovarian carcinoma xenografts with 111In-labeled monoclonal antibodies. Nucl. Med. Biol. 2010, 37, 957–964. [Google Scholar] [CrossRef]
- Price, E.W.; Zeglis, B.M.; Cawthray, J.F.; Ramogida, C.F.; Ramos, N.; Lewis, J.S.; Adam, M.J.; Orvig, C. H(4)octapa-trastuzumab: Versatile acyclic chelate system for 111In and 177Lu imaging and therapy. J. Am. Chem. Soc. 2013, 135, 12707–12721. [Google Scholar] [CrossRef]
- Yoshida, H.; Mochizuki, M.; Kainouchi, M.; Ishida, T.; Sakata, K.; Yokoyama, S.; Hoshino, T.; Takezawa, M.; Matsumoto, Y.; Miyamoto, T.; et al. Clinical application of indium-111 antimyosin antibody and thallium-201 dual nuclide single photon emission computed tomography in acute myocardial infarction. Ann. Nucl. Med. 1991, 5, 41–46. [Google Scholar] [CrossRef]
- Divgi, C.R.; Welt, S.; Kris, M.; Real, F.X.; Yeh, S.D.; Gralla, R.; Merchant, B.; Schweighart, S.; Unger, M.; Larson, S.M.; et al. Phase I and imaging trial of indium-111 labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J. Natl. Cancer Inst. 1991, 83, 97–104. [Google Scholar] [CrossRef]
- Dillman, L.T.; von der Lage, F.C. NM/MIRD Pamphlet No. 10: Radionuclide Decay Schemes and Nuclear Parameters for Use in Radiation-Dose Estimation. New York. Soc. Nucl. Med. 1975, 69, 54. [Google Scholar]
- Bading, J.R.; Hörling, M.; Williams, L.E.; Colcher, D.; Raubitschek, A.; Strand, S.E. Quantitative serial imaging of an 124I anti-CEA monoclonal antibody in tumor-bearing mice. Cancer Biother. Radiopharm. 2008, 23, 399–409. [Google Scholar] [CrossRef]
- Yao, M.; Faulhaber, P.F. PET imaging of the head and neck. PET Clinics 2012, 7, 450. [Google Scholar]
- Lee, F.T.; Hall, C.; Rigopoulos, A.; Zweit, J.; Pathmaraj, K.; O’Keefe, G.J.; Smyth, F.E.; Welt, S.; Old, L.J.; Scott, A.M. Immuno-PET of human colon xenograft- bearing BALB/c nude mice using 124I-CDR-grafted humanized A33 monoclonal antibody. J. Nucl. Med. 2001, 42, 764–769. [Google Scholar]
- Fortin, M.A.; Salnikov, A.V.; Nestor, M.; Heldin, N.E.; Rubin, K.; Lundqvist, H. Immuno-PET of undifferentiated thyroid carcinoma with radioiodine-labelled antibody cMAb U36: Application to antibody tumour uptake studies. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1376–1387. [Google Scholar] [CrossRef]
- Lee, F.T.; O’Keefe, G.J.; Gan, H.K.; Mountain, A.J.; Jones, G.R.; Saunder, T.H.; Sagona, J.; Rigopoulos, A.; Smyth, F.E.; Johns, T.G.; et al. Immuno-PET quantitation of de2-7 epidermal growth factor receptor expression in glioma using 124I-IMP-R4-labeled antibody ch806. J. Nucl. Med. 2010, 51, 967–972. [Google Scholar] [CrossRef]
- Tijink, B.M.; Neri, D.; Leemans, C.R.; Budde, M.; Dinkelborg, L.M.; Stigter-van Walsum, M.; Zardi, L.; van Dongen, G.A. Radioimmunotherapy of head and neck cancer xenografts using 131I-labeled antibody L19-SIP for selective targeting of tumor vasculature. J. Nucl. Med. 2006, 47, 1127–1135. [Google Scholar]
- Nestor, M.; Ekberg, T.; Dring, J.; van Dongen, G.A.; Wester, K.; Tolmachev, V.; Anniko, M. Quantification of CD44v6 and EGFR expression in head and neck squamous cell carcinomas using a single-dose radioimmunoassay. Tumour Biol. 2007, 28, 253–263. [Google Scholar] [CrossRef]
- Nordberg, E.; Friedman, M.; Göstring, L.; Adams, G.P.; Brismar, H.; Nilsson, F.Y.; Ståhl, S.; Glimelius, B.; Carlsson, J. Cellular studies of binding, internalization and retention of a radiolabeled EGFR-binding affibody molecule. Nucl. Med. Biol. 2007, 34, 609–618. [Google Scholar] [CrossRef]
- Hoeben, B.A.; Molkenboer-Kuenen, J.D.; Oyen, W.J.; Peeters, W.J.; Kaanders, J.H.; Bussink, J.; Boerman, O.C. Radiolabeled cetuximab: Dose optimization for epidermal growth factor receptor imaging in a head-and-neck squamous cell carcinoma model. Int. J. Cancer 2011, 129, 870–878. [Google Scholar] [CrossRef]
- Tijink, B.M.; Laeremans, T.; Budde, M.; Stigter-van Walsum, M.; Dreier, T.; de Haard, H.J.; Leemans, C.R.; van Dongen, G.A. Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: Taking advantage of modular Nanobody technology. Mol. Cancer Ther. 2008, 7, 2288–2297. [Google Scholar] [CrossRef]
- Börjesson, P.K.; Postema, E.J.; de Bree, R.; Roos, J.C.; Leemans, C.R.; Kairemo, K.J.; van Dongen, G.A. Radioimmunodetection and radioimmunotherapy of head and neck cancer. Oral. Oncol. 2004, 40, 761–772. [Google Scholar] [CrossRef]
- Jowsey, J.; Rowland, R.E.; Marshall, J.H. The deposition of the rare earths in bone. Radiat. Res. 1958, 8, 490–501. [Google Scholar] [CrossRef]
- Minarik, D.; Ljungberg, M.; Segars, P.; Gleisner, K.S. Evaluation of quantitative planar 90Y bremsstrahlung whole-body imaging. Phys. Med. Biol. 2009, 54, 5873–5883. [Google Scholar] [CrossRef]
- Elschot, M.; Vermolen, B.J.; Lam, M.G.; de Keizer, B.; van den Bosch, M.A.; de Jong, H.W. Quantitative comparison of PET and Bremsstrahlung SPECT for imaging the in vivo yttrium-90 microsphere distribution after liver radioembolization. PLoS One 2013, 8, e55742. [Google Scholar]
- Goodwin, D.A.; Meares, C.F.; Watanabe, N.; McTigue, M.; Chaovapong, W.; Ransone, C.M.; Renn, O.; Greiner, D.P.; Kukis, D.L.; Kronenberger, S.I. Pharmacokinetics of pretargeted monoclonal antibody 2D12.5 and 88Y-Janus-2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) in BALB/c mice with KHJJ mouse adenocarcinoma: A model for 90Y radioimmunotherapy. Cancer Res. 1994, 54, 5937–5946. [Google Scholar]
- Postema, E.J.; Frielink, C.; Oyen, W.J.; Raemaekers, J.M.; Goldenberg, D.M.; Corstens, F.H.; Boerman, O.C. Biodistribution of 131I-, 186Re-, 177Lu-, and 88Y-labeled hLL2 (Epratuzumab) in nude mice with CD22-positive lymphoma. Cancer Biother. Radiopharm. 2003, 18, 525–533. [Google Scholar] [CrossRef]
- Walrand, S.; Flux, G.D.; Konijnenberg, M.W.; Valkema, R.; Krenning, E.P.; Lhommel, R.; Pauwels, S.; Jamar, F. Dosimetry of yttrium-labelled radiopharmaceuticals for internal therapy: 86Y or 90Y imaging? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, S57–S68. [Google Scholar] [CrossRef]
- Niu, G.; Sun, X.; Cao, Q.; Courter, D.; Koong, A.; Le, Q.T.; Gambhir, S.S.; Chen, X. Cetuximab-based immunotherapy and radioimmunotherapy of head and neck squamous cell carcinoma. Clin. Cancer Res. 2010, 16, 2095–2105. [Google Scholar] [CrossRef]
- Koi, L.; Bergmann, R.; Brüchner, K.; Pietzsch, H.J.; Krause, M.; Steinbach, J.; Zips, D.; Baumann, M. Theragnostic radiolabeled EGFR-antibody improves local tumor control after external radiotherapy. Radiother. Oncol. 2014, in press. [Google Scholar]
- Saki, M.; Toulany, M.; Sihver, W.; Zenker, M.; Heldt, J.M.; Mosch, B.; Pietzsch, H.J.; Baumann, M.; Steinbach, J.; Rodemann, H.P. Cellular and molecular properties of 90Y-labeled cetuximab in combination with radiotherapy on human tumor cells in vitro. Strahlenther. Onkol. 2012, 188, 823–832. [Google Scholar] [CrossRef]
- Verburg, F.A.; Luster, M.; Lassmann, M.; Reiners, C. 131I therapy in patients with benign thyroid disease does not conclusively lead to a higher risk of subsequent malignancies. Nuklearmedizin 2011, 50, 93–99. [Google Scholar]
- Grünwald, F.; Ezziddin, S. 131I-metaiodobenzylguanidine therapy of neuroblastoma and other neuroendocrine tumors. Semin. Nuc. Med. 2010, 40, 153–163. [Google Scholar] [CrossRef]
- Sisson, J.C.; Carey, J.E. Thyroid carcinoma with high levels of function: Treatment with 131I. J. Nucl. Med. 2001, 42, 975–983. [Google Scholar]
- Xue, Y.L.; Qiu, Z.L.; Song, H.J.; Luo, Q.Y. Value of 131I SPECT/CT for the evaluation of differentiated thyroid cancer: A systematic review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 768–778. [Google Scholar] [CrossRef]
- Rades, D.; Wolff, C.; Nadrowitz, R.; Breunig, C.; Schild, S.E.; Baehre, M.; Meller, B. Radioactive EGFR antibody cetuximab in multimodal cancer treatment: Stability and synergistic effects with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1226–1231. [Google Scholar] [CrossRef]
- Schlom, J.; Siler, K.; Milenic, D.E.; Eggensperger, D.; Colcher, D.; Miller, L.S.; Houchens, D.; Cheng, R.; Kaplan, D.; Goeckeler, W. Monoclonal antibody-based therapy of a human tumor xenograft with a 177lutetium-labeled im munoconjugate. Cancer Res. 1991, 51, 2889–2896. [Google Scholar]
- Mulligan, T.; Carrasquillo, J.A.; Chung, Y.; Milenic, D.E.; Schlom, J.; Feuerstein, I.; Paik, C.; Perentesis, P.; Reynolds, J.; Curt, G.; et al. Phase I study of intravenous Lu-labeled CC49 murine monoclonal antibody in patients with advanced adenocarcinoma. J. Clin. Cancer Res. 1995, 1, 1447–1454. [Google Scholar]
- Stein, R.; Govindan, S.V.; Chen, S.; Reed, L.; Richel, H.; Griffiths, G.L.; Hansen, H.J.; Goldenberg, D.M. Radioimmunotherapy of a human lung cancer xenograft with monoclonal antibody RS7: Evaluation of 177Lu and comparison of its efficacy with that of 90Y and residualizing 131I. J. Nucl. Med. 2001, 42, 967–974. [Google Scholar]
- Lee, S.Y.; Hong, Y.D.; Kim, H.S.; Choi, S.J. Synthesis and application of a novel cysteine-based DTPA-NCS for targeted radioimmunotherapy. Nucl. Med. Biol. 2013, 40, 424–429. [Google Scholar] [CrossRef]
- Jurcic, J.G.; Larson, S.M.; Sgouros, G.; McDevitt, M.R.; Finn, R.D.; Divgi, C.R.; Ballangrud, A.M.; Hamacher, K.A.; Ma, D.; Humm, J.L.; et al. Targeted alpha particle immunotherapy for myeloid leukemia. Blood 2002, 100, 1233–1239. [Google Scholar]
- Song, H.; Shahverdi, K.; Huso, D.L.; Esaias, C.; Fox, J.; Liedy, A.; Zhang, Z.; Reilly, R.T.; Apostolidis, C.; Morgenstern, A.; et al. 213Bi (alpha-emitter)-antibody targeting of breast cancer metastases in the neu-N transgenic mouse model. Cancer Res. 2008, 68, 3873–3880. [Google Scholar] [CrossRef]
- Ma, D.; McDevitt, M.R.; Finn, R.D.; Scheinberg, D.A. Breakthrough of 225Ac and its radionuclide daughters from an 225Ac/213Bi generator: Development of new methods, quantitative characterization, and implications for clinical use. Appl. Radiat. Isot. 2001, 55, 667–678. [Google Scholar] [CrossRef]
- Rosenblat, T.L.; McDevitt, M.R.; Mulford, D.A.; Pandit-Taskar, N.; Divgi, C.R.; Panageas, K.S.; Heaney, M.L.; Chanel, S.; Morgenstern, A.; Sgouros, G.; et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin. Cancer Res. 2010, 16, 5303–5311. [Google Scholar] [CrossRef]
- Andersson, H.; Cederkrantz, E.; Bäck, T.; Divgi, C.; Elgqvist, J.; Himmelman, J.; Horvath, G.; Jacobsson, L.; Jensen, H.; Lindegren, S.; et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: Pharmacokinetics and dosimetry of 211At-MX35 F(ab')2—A phase I study. J. Nucl. Med. 2009, 50, 1153–1160. [Google Scholar] [CrossRef]
- Allen, B.J.; Singla, A.A.; Rizvi, S.M.; Graham, P.; Bruchertseifer, F.; Apostolidis, C.; Morgenstern, A. Analysis of patient survival in a Phase I trial of systemic targeted α-therapy for metastatic melanoma. Immunotherapy 2011, 3, 1041–1050. [Google Scholar] [CrossRef]
- Song, H.; Hedayati, M.; Hobbs, R.F.; Shao, C.; Bruchertseifer, F.; Morgenstern, A.; Deweese, T.L.; Sgouros, G. Targeting aberrant DNA double strand break repair in triple negative breast cancer with alpha particle emitter radiolabeled anti-EGFR antibody. Mol. Cancer Ther. 2013, 12, 2043–2054. [Google Scholar] [CrossRef]
- Brechbiel, M.W. Bifunctional chelates for metal nuclides. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 166–173. [Google Scholar]
- Pritchard, J.H.; Ackerman, M.; Tubis, M.; Blahd, W.H. Indium-111-labeled antibody heavy metal chelate conjugates: A potential alternative to radioiodination. Proc. Soc. Exp. Biol. Med. 1976, 151, 297–302. [Google Scholar] [CrossRef]
- Ward, M.C.; Roberts, K.R.; Babich, J.W.; Bukhari, M.A.; Coghlan, G.; Westwood, J.H.; McCready, V.R.; Ott, R.J. An antibody-desferrioxamine conjugate labelled with 67Ga. Int. J. Rad. Appl. Instrum. B 1986, 13, 505–307. [Google Scholar] [CrossRef]
- Perk, L.R.; Vosjan, M.J.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. p-Isothiocyanatobenzyl-desferrioxamine: A new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 250–259. [Google Scholar] [CrossRef]
- Chang, A.J.; de Silva, R.A.; Lapi, S.E. Development and characterization of 89Zr-labeled panitumumab for immuno-positron emission tomographic imaging of the epidermal growth factor receptor. Mol. Imaging 2013, 12, 17–27. [Google Scholar]
- Aerts, H.J.; Dubois, L.; Perk, L.; Vermaelen, P.; van Dongen, G.A.; Wouters, B.G.; Lambin, P. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J. Nucl. Med. 2009, 50, 123–131. [Google Scholar]
- Karmani, L.; Labar, D.; Valembois, V.; Bouchat, V.; Nagaswaran, P.G.; Bol, A.; Gillart, J.; Levêque, P.; Bouzin, C.; Bonifazi, D.; et al. Antibody-functionalized nanoparticles for imaging cancer: Influence of conjugation to gold nanoparticles on the biodistribution of 89Zr-labeled cetuximab in mice. Contrast Media Mol. Imaging 2013, 8, 402–408. [Google Scholar] [CrossRef]
- McMurry, T.J.; Pippin, C.G.; Wu, C.; Deal, K.A.; Brechbiel, M.W.; Mirzadeh, S.; Gansow, O.A. Physical parameters and biological stability of yttrium(III) diethylenetriaminepentaacetic acid derivative conjugates. J. Med. Chem. 1998, 41, 3546–3549. [Google Scholar] [CrossRef]
- Kobayashi, H.; Wu, C.; Yoo, T.M.; Sun, B.F.; Drumm, D.; Pastan, I.; Paik, C.H.; Gansow, O.A.; Carrasquillo, J.A.; Brechbiel, M.W. Evaluation of the in vivo biodistribution of yttrium-labeled isomers of CHX-DTPA-conjugated monoclonal antibodies. J. Nucl. Med. 1998, 39, 829–836. [Google Scholar]
- Lee, F.T.; Mountain, A.J.; Kelly, M.P.; Hall, C.; Rigopoulos, A.; Johns, T.G.; Smyth, F.E.; Brechbiel, M.W.; Nice, E.C.; Burgess, A.W.; et al. Enhanced efficacy of radioimmunotherapy with 90Y-CHX-A′′-DTPA-hu3S193 by inhibition of epidermal growth factor receptor (EGFR) signaling with EGFR tyrosine kinase inhibitor AG1478. Clin. Cancer Res. 2005, 11, 7080s–7086s. [Google Scholar] [CrossRef]
- Fani, M.; Bouziotis, P.; Harris, A.L.; Psimadas, D.; Gourni, E.; Loudos, G.; Varvarigou, A.D.; Maecke, H.R. 177Lu-labeled-VG76e monoclonal antibody in tumor angiogenesis: A comparative study using DOTA and DTPA chelating systems. Radiochim. Acta 2007, 95, 351–357. [Google Scholar]
- Ray, G.L.; Baidoo, K.E.; Wong, K.J.; Williams, M.; Garmestani, K.; Brechbiel, M.W.; Milenic, D.E. Preclinical evaluation of a monoclonal antibody targeting the epidermal growth factor receptor as a radioimmunodiagnostic and radioimmunotherapeutic agent. Br. J. Pharmacol. 2009, 157, 1541–1548. [Google Scholar] [CrossRef]
- Boswell, C.A.; Sun, X.; Niu, W.; Weisman, G.R.; Wong, E.H.; Rheingold, A.L.; Anderson, C.J. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J. Med. Chem. 2004, 47, 1465–1474. [Google Scholar] [CrossRef]
- Tolmachev, V.; Wållberg, H.; Andersson, K.; Wennborg, A.; Lundqvist, H.; Orlova, A. The influence of Bz-DOTA and CHX-A′′-DTPA on the biodistribution of ABD-fused anti-HER2 Affibody molecules: implications for 114mIn-mediated targeting therapy. Eur. J. Nucl. Med. Mol. Imaging. 2009, 36, 1460–1468. [Google Scholar] [CrossRef]
- Milenic, D.E.; Garmestani, K.; Chappell, L.L.; Dadachova, E.; Yordanov, A.; Ma, D.; Schlom, J.; Brechbiel, M.W. In vivo comparison of macrocyclic and acyclic ligands for radiolabeling of monoclonal antibodies with 177Lu for radioimmunotherapeutic applications. Nucl. Med. Biol. 2002, 29, 431–442. [Google Scholar] [CrossRef]
- Niu, G.; Cai, W.; Chen, K.; Chen, X. Non-invasive PET imaging of EGFR degradation induced by a heat shock protein 90 inhibitor. Mol. Imaging Biol. 2008, 10, 99–106. [Google Scholar] [CrossRef]
- Delgado, R.; Sun, Y.; Motekaitis, R.J.; Martell, A.E. Stabilities of divalent and trivalent metal ion complexes of macrocyclic triazatriacetic acids. Inorg. Chem. 1993, 32, 3320–3326. [Google Scholar] [CrossRef]
- Sprague, J.E.; Peng, Y.; Sun, X.; Weisman, G.R.; Wong, E.H.; Achilefu, S.; Anderson, C.J. Preparation and biological evaluation of copper-64-labeled Tyr3-Octreotate using a cross-bridged macrocyclic cheator. Clin. Cancer Res. 2004, 10, 8674–8682. [Google Scholar] [CrossRef]
- Ingargiola, M.; Dittfeld, C.; Runge, R.; Zenker, M.; Heldt, J.M.; Steinbach, J.; Cordes, N.; Baumann, M.; Kotzerke, J.; Kunz-Schughart, L.A. Flow cytometric cell-based assay to preselect antibody constructs for radionuclide conjugation. Cytometry A 2012, 81, 865–873. [Google Scholar]
- Zhang, Y.; Hong, H.; Engle, J.W.; Yang, Y.; Theuer, C.P.; Barnhart, T.E.; Cai, W. Positron Emission Tomography and Optical Imaging of Tumor CD105 Expression with a Dual-Labeled Monoclonal Antibody. Mol. Pharm. 2012, 9, 645–653. [Google Scholar] [CrossRef]
- Cohen, R.; Stammes, M.A.; de Roos, I.H.; Stigter-van Walsum, M.; Visser, G.W.; van Dongen, G.A. Inert coupling of IRDye800CW to monoclonal antibodies for clinical optical imaging of tumor targets. EJNMMI Res. 2011, 1, 31. [Google Scholar] [CrossRef]
- Vakili, A.; Jalilian, A.R.; Yavari, K.; Shirvani-Arani, S.; Khanchi, A.; Bahrami-Samani, A.; Salimi, B.; Khorrami-Moghadam, A. Preparation and quality control and biodistribution studies of [90Y]-DOTA-cetuximab for radioimmunotherapy. J. Radioanal. Nucl. Chem. 2013, 296, 1287–1294. [Google Scholar] [CrossRef]
- Wen, X.; Wu, Q.P.; Ke, S.; Ellis, L.; Charnsangavej, C.; Delpassand, A.S.; Wallace, S.; Li, C. Conjugation with 111In-DTPA-poly(ethylene glycol) improves imaging of anti-EGF receptor antibody C225. J. Nucl. Med. 2001, 42, 1530–1537. [Google Scholar]
- Van Dijk, L.K.; Hoeben, B.A.; Stegeman, H.; Kaanders, J.H.; Franssen, G.M.; Boerman, O.C.; Bussink, J. 111In-cetuximab-F(ab')2 SPECT imaging for quantification of accessible epidermal growth factor receptors (EGFR) in HNSCC xenografts. Radiother. Oncol. 2013, 108, 484–488. [Google Scholar] [CrossRef]
- Van Dongen, G.A.; Visser, G.W.; Lub-de Hooge, M.N.; de Vries, E.G.; Perk, L.R. Immuno-PET: A navigator in monoclonal antibody development and applications. Oncologist 2007, 12, 1379–1389. [Google Scholar] [CrossRef]
- Walrand, S.; Barone, R.; Pauwels, S.; Jamar, F. Experimental facts supporting a red marrow uptake due to radiometal transchelation in 90Y-DOTATOC therapy and relationship to the decrease of platelet counts. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1270–1280. [Google Scholar] [CrossRef]
- Vakili, A.; Jalilian, A.R.; Moghadam, A.K.; Ghazi-Zahedi, M.; Salimi, B. Evaluation and comparison of human absorbed dose of 90Y-DOTA-Cetuximab in various age groups based on distribution data in rats. J. Med. Phys. 2012, 37, 226–234. [Google Scholar] [CrossRef]
- Pilaro, A.M. Pharmacology/toxicology review and evaluation. Erbitux. Accessdata FDA Application number STN/BLA 125084. Cent. Drug Eval. Res. 2003, 1, 1–32. [Google Scholar]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug. Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Ogawa, M.; Regino, C.A.; Choyke, P.L.; Kobayashi, H. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol. Cancer Ther. 2009, 8, 232–239. [Google Scholar] [CrossRef]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef]
- Perera, R.M.; Zoncu, R.; Johns, T.G.; Pypaert, M.; Lee, F.T.; Mellman, I.; Old, L.J.; Toomre, D.K.; Scott, A.M. Internalization, intracellular trafficking, and biodistribution of monoclonal antibody 806: A novel anti-epidermal growth factor receptor antibody. Neoplasia 2007, 9, 1099–1110. [Google Scholar] [CrossRef]
- Oude Munnink, T.H.; Tamas, K.R.; Lub-de Hooge, M.N.; Vedelaar, S.R.; Timmer-Bosscha, H.; Walenkamp, A.M.; Weidner, K.M.; Herting, F.; Tessier, J.; de Vries, E.G. Placental growth factor (PlGF)-specific uptake in tumor microenvironment of 89Zr-labeled PlGF antibody RO5323441. J. Nucl. Med. 2013, 54, 929–935. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Dittmann, K.; Mayer, C.; Rodemann, H.P. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother. Oncol. 2005, 76, 157–161. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol. Ther. 2009, 8, 1994–2001. [Google Scholar]
- Saker, J.; Kriegs, M.; Zenker, M.; Heldt, J.M.; Eke, I.; Pietzsch, H.J.; Grénman, R.; Cordes, N.; Petersen, C.; Baumann, M.; et al. Inactivation of HNSCC cells by 90Y-labeled cetuximab strictly depends on the number of induced DNA double-strand breaks. J. Nucl. Med. 2013, 54, 416–423. [Google Scholar] [CrossRef]
- Krause, M.; Ostermann, G.; Petersen, C.; Yaromina, A.; Hessel, F.; Harstrick, A.; van der Kogel, A.J.; Thames, H.D.; Baumann, M. Decreased repopulation as well as increased reoxygenation contribute to the improvement in local control after targeting of the EGFR by C225 during fractionated irradiation. Radiother. Oncol. 2005, 76, 162–167. [Google Scholar] [CrossRef]
- Eiblmaier, M.; Meyer, L.A.; Anderson, C.J. The role of p53 in the trafficking of copper-64 to tumor cell nuclei. Cancer Biol. Ther. 2008, 7, 63–69. [Google Scholar] [CrossRef]
- Rades, D.; Nadrowitz, R.; Buchmann, I.; Hunold, P.; Noack, F.; Schild, S.E.; Meller, B. Radiolabeled cetuximab plus whole-brain irradiation (WBI) for the treatment of brain metastases from non-small cell lung cancer (NSCLC). Strahlenther. Onkol. 2010, 186, 458–462. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sihver, W.; Pietzsch, J.; Krause, M.; Baumann, M.; Steinbach, J.; Pietzsch, H.-J. Radiolabeled Cetuximab Conjugates for EGFR Targeted Cancer Diagnostics and Therapy. Pharmaceuticals 2014, 7, 311-338. https://doi.org/10.3390/ph7030311
Sihver W, Pietzsch J, Krause M, Baumann M, Steinbach J, Pietzsch H-J. Radiolabeled Cetuximab Conjugates for EGFR Targeted Cancer Diagnostics and Therapy. Pharmaceuticals. 2014; 7(3):311-338. https://doi.org/10.3390/ph7030311
Chicago/Turabian StyleSihver, Wiebke, Jens Pietzsch, Mechthild Krause, Michael Baumann, Jörg Steinbach, and Hans-Jürgen Pietzsch. 2014. "Radiolabeled Cetuximab Conjugates for EGFR Targeted Cancer Diagnostics and Therapy" Pharmaceuticals 7, no. 3: 311-338. https://doi.org/10.3390/ph7030311