A Great Barrier Reef Sinularia sp. Yields Two New Cytotoxic Diterpenes
Abstract
:1. Introduction
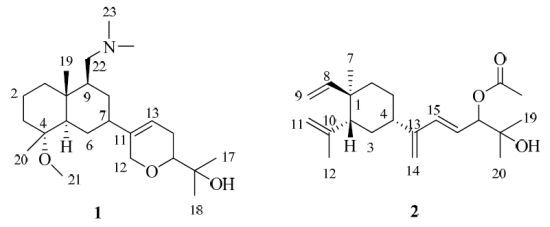
2. Results and Discussion
| No. | 13C δ (m) | 1H δ (m, J Hz) | COSY | gHMBC | nOe |
|---|---|---|---|---|---|
| 1 | 42.3 (t) | 1.84 (1H, m) | Hb-1, Hb-2 | C-10, C-5, C-9, C-2, C-3 | |
| 1.24 (1H, m) | Ha-1, Ha-2, Hb-2 | C-5, C-19, C-2 | |||
| 2 | 28.1 (t) | 1.59 (1H, m ) | Ha-1, Ha-3 | C-4 | |
| 1.45 (1H, m) | Ha-1 | C-10, C-1, C-4 | |||
| 3 | 36.3 (t) | 1.85 (1H, m) | Ha-2, Hb-3 | C-5, C-1, C-4, C-20 | |
| 1.60 (1H, m) | Hb-2, Ha-3 | C-5, C-1, C-4 | |||
| 4 | 76.8 (s) | ||||
| 5 | 53.1 (d) | 1.49 (1H, m) | H2-6 | C-6, C-19, C-4, C-20 | |
| 6 | 26.7 (t) | 1.82 (2H, m) | H-5, H-7 | C-10, C-8 | |
| 7 | 43.1 (d) | 1.86(1H, m) | H2-6 | C-5, C-8, C-9 | |
| 8 | 25.0 (t) | 1.85 (1H, m) | Hb-8, H-9 | ||
| 1.52 (1H, m) | Ha-8 | C-10, C-7, C-22 | |||
| 9 | 45.2 (d) | 1.52 (1H, m) | Ha-22, Hb-22 | C-10, C-7, C-22 | |
| 10 | 38.0 (s) | ||||
| 11 | 142.5 (s) | ||||
| 12 | 69.0 (t) | 4.16 (2H, brs) | H-13 | C-7, C-11, C-13, C-15 | |
| 13 | 117.8(d) | 5.59 (1H, d, 5.2) | H-12, Ha-14, Hb-14 | C-7, C-12, C-14, C-15 | |
| 14 | 26.3 (t) | 2.12 (1H, m) | H-13, Hb-14, H-15 | ||
| 2.00 (1H, m) | H-13, Ha-14, H-15 | ||||
| 15 | 81.9 (d) | 3.25 (1H, m) | Ha-14, Hb-14 | C-17, C-18 | |
| 16 | 72.7 (s) | ||||
| 17 | 25.2 (q) | 1.17 (3H, s) | C-15, C-16, C-18 | ||
| 18 | 25.6 (q) | 1.17 (3H, s) | C-15, C-16, C-17 | ||
| 19 | 15.2 (q) | 0.89 (3H, s) | Hb-1, Hb-2, H-20, H2-22 | ||
| 20 | 18.7 (q) | 1.08 (3H, s) | C-5, C-4, C-3 | Hb-1, Hb-2, H-19 | |
| 21 | 48.1 (q) | 3.16 (3H, s) | C-4 | H-5 | |
| 22 | 60.5 (t) | 3.25 (1H, dd, 3.2, 11.0) | H-9, Hb-22 | C-10, C-8, C-9 | |
| 2.93 (1H, dd, 11.0, 13.1) | H-9, Ha-22 | C-9 | |||
| 23 | 45.2 (q) | 2.90 (3H, s) | C-22, C-24 | ||
| 24 | 45.2 (q) | 2.90 (3H, s) | C-22, C-23 |


| No. | 13C δ (m) | 1H δ (m, J Hz) | COSY | gHMBC | nOe |
|---|---|---|---|---|---|
| 1 | 41.0 (s) | ||||
| 2 | 54.1(d) | 2.13 (1H, m) | H-3 | C-1, C-4, C-7, C-10, C-11, C-12 | H-4, H-12 |
| 3 | 35.2 (t) | 1.66 (2H, m) | H-2, H-4 | C-2, C-4 | |
| 4 | 41.5 (d) | 2.31 (1H, tdd, 3.4, 4.2, 11.7) | H-3, Ha-5, Hb-5 | C-3, C-13, C-14 | H-2, Ha-5, Hb-5, Ha-6, H-14, H-15, H-16, H-19, H-20, O-CO-CH3 |
| 5 | 28.8 (t) | 1.64 (1H, m) | H-4, Hb-5, Ha-6 | C-3 | H-4 |
| 1.52 (1H, m) | H-4, Ha-5 | C-1, C-4 | H-2, H-4 | ||
| 6 | 41.2 (t) | 1.60 (1H, m) | Ha-5, Hb-6 | C-1, C-2, C-4, C-5, C-7 | H-4, H-12 |
| 1.45 (1H, m) | Ha-6 | C-2, C-4, C-5 | |||
| 7 | 17.1 (q) | 1.03 (3H, s) | C-1, C-2, C-6, C-8 | H-12 | |
| 8 | 151.6 (d) | 5.87 (1H, dd, 10.8, 17.6) | Ha-9 | C-1, C-2, C-6, C-7 | H-12 |
| 9 | 110.4 (t) | 4.93 (1H, dd, 1.2. 17.6) | H-8, Hb-9 | C-1, C-2, C-8 | H-12, H-17 |
| 4.90 (1H, dd, 1.2, 10.8) | H-8, Hb-9 | C-1, C-2, C-8 | H-17 | ||
| 10 | 148.9 (s) | ||||
| 11 | 112.7 (t) | 4.81 (1H, brt, 1.5) | Hb-11, H3-12 | C-1, C-2, C-10, C-12 | H-12 |
| 4.59 (1H, brs) | Ha-11, H3-12 | C-1, C-2, C-10, C-12 | H-12 | ||
| 12 | 25.3 (q) | 1.71 (3H, brs) | Ha-11, Hb-11 | C-1, C-2, C-10, C-11 | H-2, Ha-6, H-7, H-8, Ha-9, Ha-11, Hb-11, H-19 |
| 13 | 151.9 (s) | ||||
| 14 | 114.3 (t) | 5.06 (2H, s) | C-4, C-13, C-15, C-16 | H-4 | |
| 15 | 137.4(d) | 6.26 (1H, d, 16.0) | H-16 | C-4, C-13, C-14, C-16, C-17 | H-4, H-17 |
| 16 | 124.4 (d) | 5.78 (1H, dd, 7.6, 16.0) | H-15, H-17 | C-13,C-14, C-17, C-18 | H-4, H-17 |
| 17 | 82.2 (d) | 5.14 (1H, brd, 7.6) | H-16 | C-15, C-16, C-18, C-19, C-20, O-CO-CH3 | Ha-9, Hb-9, H-15, H-16, H-19, H-20, O-CO-CH3 |
| 18 | 72.7 (s) | ||||
| 19 | 25.6 (q) | 1.17 (3H, s) | C-17, C-18, C-20 | H-4, H-12, H-17, O-CO-CH3 | |
| 20 | 26.2 (q) | 1.18 (3H, s) | C-17, C-18, C-19 | H-4, H-17, O-CO-CH3 | |
| O-CO-CH3 | 172.1 (s) | ||||
| O-CO-CH3 | 21.1 (q) | 2.09 (3H, s) | OAc | H-4, H-7, H-17, H-19, H-20 |
| Compound | SF-268 a | MCF-7 b | H460 c |
|---|---|---|---|
| 1 | 175 | 70 | 125 |
| 2 | 15 | 8.8 | 11.5 |
| Loba-8,10,13(15)-triene-16,17,18-triol | 18.5 | 17 | 13 |
| Lobatrientriol | NT | NT | NT |
| 14,17-Epoxyloba-8,10,13(15)-trien-18-ol-18-acetate | 14 | 16 | 18.5 |
| Lobatrienolide | 7.4 | 17 | 18 |
| 14,18-Epoxyloba-8,10,13(15)-trien-17-ol | NT | NT | NT |
| (17R)-Loba-8,10,13(15)-trien-17,18-diol | NT | NT | NT |
| (1E,3E,7E)-11,12-Epoxycembratrien-15-ol | 6.8 | 12 | 18.5 |
| Sarcophytol-B | 16 | 12.5 | 15 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Bioassay
3.4. Extraction and Isolation
3.4.1. (4R*,5R*,9S*,10R*,11Z)-4-Methoxy-9-((dimethylamino)-methyl)-12,15-epoxy-11(13)-en-decahydronaphthalen-16-ol (1)
3.4.2. (1R*,2R*,4S*,15E)-Loba-8,10,13(14),15(16)-tetraen-17,18-diol-17-acetate (2)
3.4.3. Loba-8,10,13(15)-triene-16,17,18-triol
3.4.4. Lobatrientriol
3.4.5. 14,17-Epoxyloba-8,10,13(15)-trien-18-ol-18-acetate
3.4.6. Lobatrienolide
3.4.7. (1E,3E,7E)-11,12-Epoxycembratrien-15-ol
3.4.8. 14,18-Epoxyloba-8,10,13(15)-trien-17-ol
3.4.9. (17R)-Loba-8,10,13(15)-trien-17,18-diol
3.4.10. Sarcophytol-B
4. Conclusion
Acknowledgements
References
- Bowden, B.F.; Coll, J.C.; de Silva, E.D.; de Costa, M.S.L.; Djura, P.J.; Mahendran, M.; Tapiolas, D.M. Studies of Australian soft corals. XXXI. Novel furanosesquiterpenes from several sinularian soft corals (Coelenterata, Octocorallia, Alcyonacea). Aust. J. Chem. 1983, 36, 371–376. [Google Scholar] [CrossRef]
- Park, S.K.; Scheuer, P.J. Isolation and structure determination of two furanosesquiterpenes from the soft coral Sinularia lochmodes. J. Korean Chem. Soc. 1994, 38, 749–752. [Google Scholar]
- Radwan, M.M.; Manly, S.P.; Sayed, K.A.E.; Wali, V.B.; Sylvester, P.W.; Awate, B.; Shah, G.; Ross, S.A. Sinulodurins A and B, antiproliferative and anti-invasive diterpenes from the soft coral Sinularia dura. J. Nat. Prod. 2008, 71, 1468–1471. [Google Scholar]
- Cheng, S.-Y.; Chuang, C.-T.; Wang, S.-K.; Wen, Z.-H.; Chiou, S.-F.; Hsu, C.-H.; Dai, C.-F.; Duh, C.-Y. Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. J. Nat. Prod. 2010, 73, 1184–1187. [Google Scholar] [CrossRef]
- Aceret, T.L.; Coll, J.C.; Uchio, Y.; Sammarco, P.W. Antimicrobial activity of the diterpenes flexibilide and sinulariolide derived from Sinularia flexibilis Quoy and Gaimard 1833 (Coelenterata: Alcyonacea, Octocorallia). Comp. Biochem. Physiol. Part C 1998, 120, 121–126. [Google Scholar]
- Chai, M.-C.; Wang, S.-K.; Dai, C.-F.; Duh, C.-Y. A cytotoxic lobane diterpene from the Formosan soft coral Sinularia inelegans. J. Nat. Prod. 2000, 63, 843–844. [Google Scholar]
- Hamada, T.; Kusumi, T.; Ishitsuka, M.O.; Kakisawa, H. Structures and absolute configurations of new lobane diterpenoids from the Okinawan soft coral Sinularia flexibilis. Chem. Lett. 1992, 21, 33–36. [Google Scholar]
- Duh, C.-Y.; Hou, R.-S. Cytotoxic cembranoids from the soft corals Sinularia gibberosa and Sarcophyton trocheliophorum. J. Nat. Prod. 1996, 59, 595–598. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Tseng, H.-K.; Sheu, J.-H.; Chiang, M.Y. Novel cytotoxic cembranoids from the soft coral Sinularia flexibilis. J. Nat. Prod. 1998, 61, 844–847. [Google Scholar]
- Su, J.-H.; Ahmed, A.F.; Sung, P.-J.; Chao, C.-H.; Kuo, Y.-H.; Sheu, J.-H. Manaarenolides A–I, diterpenoids from the soft coral Sinularia manaarensis. J. Nat. Prod. 2006, 69, 1134–1139. [Google Scholar]
- Lu, Y.; Huang, C.-Y.; Lin, Y.-F.; Wen, Z.-H.; Su, J.-H.; Kuo, Y.-H.; Chiang, M.Y.; Sheu, J.-H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008, 71, 1754–1759. [Google Scholar]
- Jagodzinska, B.M.; Trimmer, J.S.; Fenical, W.; Djerassi, C. Sterols in marine invertebrates. 51: Isolation and structure elucidation of C-18 functionalized sterols from the soft coral Sinularia dissecta. J. Org. Chem. 1985, 50, 2988–2992. [Google Scholar]
- Kumar, R.; Lakshmi, V. Two new glycosides from the soft coral Sinularia firma. Chem. Pharm. Bull. 2006, 54, 1650–1652. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Kulatheeswaran, R.; Rao, C.V. New sphingosines from two soft corals of the Andaman and Nicobar islands. Indian J. Chem. Sec. B 1996, 35, 578–580. [Google Scholar]
- Cheng, S.-Y.; Huang, K.-J.; Wang, S.-K.; Duh, C.-Y. Capilloquinol: A novel farnesyl quinol from the Dongsha Atoll soft coral Sinularia capillosa. Mar. Drugs 2011, 9, 1469–1476. [Google Scholar] [CrossRef]
- Coll, J.C.; Liyanage, N.; Stokie, G.J.; Van Altena, I.; Nemorin, J.N.E.; Sternhell, S.; Kazlauskas, R. Studies of Australian soft corals. III. A novel furanoquinol from Sinularia lochmodes. Aust. J. Chem. 1978, 31, 157–162. [Google Scholar] [CrossRef]
- Ojika, M.; Islam, M.K.; Shintani, T.; Zhang, Y.; Okamoto, T.; Sakagami, Y. Three new cytotoxic acylspermidines from the soft coral, Sinularia sp. Biosci. Biotechnol. Biochem. 2003, 67, 1410–1412. [Google Scholar] [CrossRef]
- Arepalli, S.K.; Sridhar, V.; Rao, J.V.; Kennady, P.K.; Venkateswarlu, Y. Furano-sesquiterpene from soft coral, Sinularia kavarittiensis: induces apoptosis via the mitochondrial-mediated caspase-dependent pathway in THP-1, leukemia cell line. Apotosis 2009, 14, 729–740. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Kon-ya, K.; Adachi, K.; Sakai, M.; Miki, W. Antifouling substances from a Palauan octocoral Sinularia sp. Fish. Sci. 1994, 60, 345–346. [Google Scholar]
- Slattery, M.; Hines, G.A.; Starmer, J.; Paul, V.J. Chemical signals in gametogenesis, spawning, and larval settlement and defense of the soft coral Sinularia polydactyla. Coral Reefs 1999, 18, 75–84. [Google Scholar] [CrossRef]
- Sammarco, P.W.; Coll, J.C.; Barre, S.; Willis, B. Competitive strategies of soft corals (Coelenterata: Octocorallia): Allelopathic effects on selected scleractinian corals. Coral Reefs 1983, 1, 173–178. [Google Scholar] [CrossRef]
- Coll, J.C.; Bowden, B.F.; Heaton, A.; Scheuer, P.J.; Li, M.K.W.; Clardy, J.; K., S.G.; Finer-Moore, J. Structures and possible functions of epoxypukalide and pukalide diterpenes associated with eggs of sinularian soft corals (Cnidaria, Anthozoa, Octocorallia, Alcyonacea, Alcyoniidae). J. Chem. Ecol. 1989, 15, 1177–1191. [Google Scholar] [CrossRef]
- Boyd, M.R. The NCI in-vitro anticancer drug discovery screen: Concept, implementation and operation, 1985–1995. In Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval; Teicher, B.A., Ed.; Humana Press: Totowa, NJ, USA, 1997; pp. 23–42. [Google Scholar]
- Kobayashi, M.; Nakagawa, T.; Mitsuhashi, H. Marine terpenes and terpenoids I: Structures of four cembrane-type diterpenes; from the soft coral Sarcophyton glaucum. Chem. Pharm. Bull. 1979, 27, 2382–2387. [Google Scholar] [CrossRef]
- Raju, B.L.; Subbaraju, G.V.; Rao, C.B.; Trimurtulu, G. Two new oxygenated lobanes from a soft coral of Lobophytum species of the Andaman and Nicobar coasts. J. Nat. Prod. 1993, 56, 961–966. [Google Scholar]
- Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; van Ofwegen, L. Four new bioactive lobane diterpenes of the soft coral Lobophytum pauciflorum from Mindoro, Philippines. J. Nat. Prod. 1998, 61, 358–361. [Google Scholar] [CrossRef]
- Dunlop, R.W.; Wells, R. Isolation of some novel diterpenes from a soft coral of the genus Lobophytum. Aust. J. Chem. 1979, 32, 1345–1351. [Google Scholar] [CrossRef]
- Trimurtulu, G.; Faulkner, D.J. Six new diterpene isonitriles from the sponge Acanthella cavernosa. J. Nat. Prod. 1994, 57, 501–506. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Wright, A.D.; Motti, C.A.; Tapiolas, D.M. Comosusols A–D and comosone A: Cytotoxic compounds from the brown alga Sporochnus comosus. J. Nat. Prod. 2011, 74, 739–743. [Google Scholar] [CrossRef]
- Kamel, H.N.; Slattery, M. Terpenoids of Sinularia: Chemistry and biomedical applications. Pharm. Biol. 2005, 43, 253–269. [Google Scholar]
- Samples Availability: Available from the authors.
Supplementary Files
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wright, A.D.; Nielson, J.L.; Tapiolas, D.M.; Liptrot, C.H.; Motti, C.A. A Great Barrier Reef Sinularia sp. Yields Two New Cytotoxic Diterpenes. Mar. Drugs 2012, 10, 1619-1630. https://doi.org/10.3390/md10081619
Wright AD, Nielson JL, Tapiolas DM, Liptrot CH, Motti CA. A Great Barrier Reef Sinularia sp. Yields Two New Cytotoxic Diterpenes. Marine Drugs. 2012; 10(8):1619-1630. https://doi.org/10.3390/md10081619
Chicago/Turabian StyleWright, Anthony D., Jonathan L. Nielson, Dianne M. Tapiolas, Catherine H. Liptrot, and Cherie A. Motti. 2012. "A Great Barrier Reef Sinularia sp. Yields Two New Cytotoxic Diterpenes" Marine Drugs 10, no. 8: 1619-1630. https://doi.org/10.3390/md10081619