The Effect of Sulfated (1→3)-α-l-Fucan from the Brown Alga Saccharina cichorioides Miyabe on Resveratrol-Induced Apoptosis in Colon Carcinoma Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of the Fucoidan from S. cichorioides
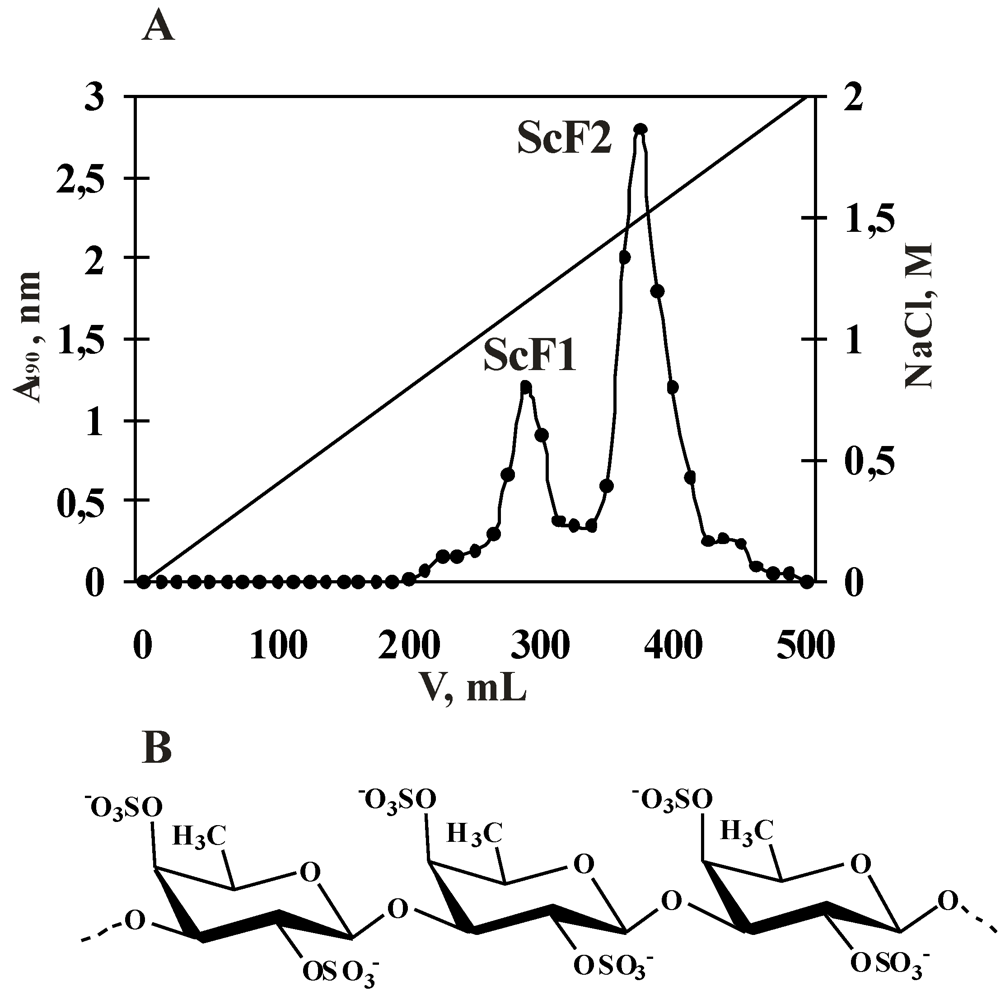
| Fraction | Yield *, % | Mw, kDa | Content **, % | Monosaccharide composition, mol% | ||
|---|---|---|---|---|---|---|
| Carbohydrate | SO3Na− | Fuc | Man | |||
| ScF1 | 1.0 | n.d. | 55.0 | 21.0 | 95.0 | 5.0 |
| ScF2 | 2.2 | 540 | 32.0 | 39.0 | 100.0 | 0 |
2.2. Anti-Tumor Activity
2.2.1. Cytotoxicity of the Fucoidan from the Brown Alga Saccharina cichorioides and Resveratrol
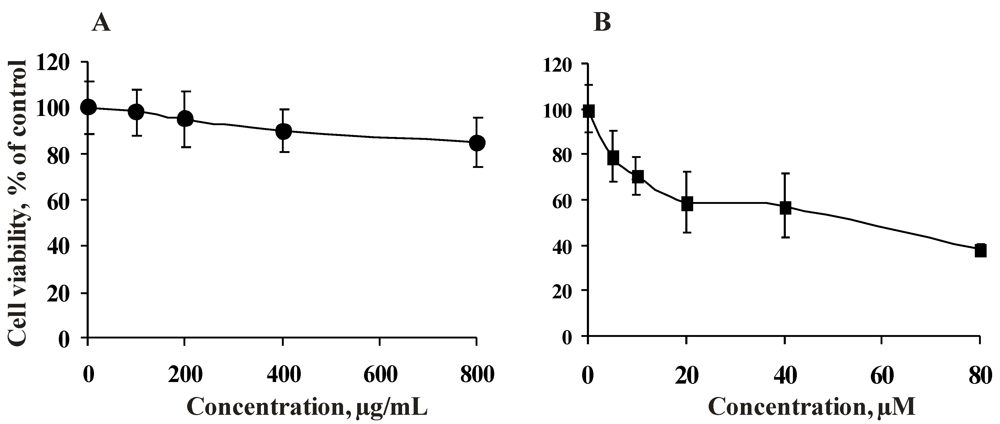
2.2.2. Antiproliferative Activity of the Fucoidan from the Brown Alga Saccharina cichorioides and Resveratrol
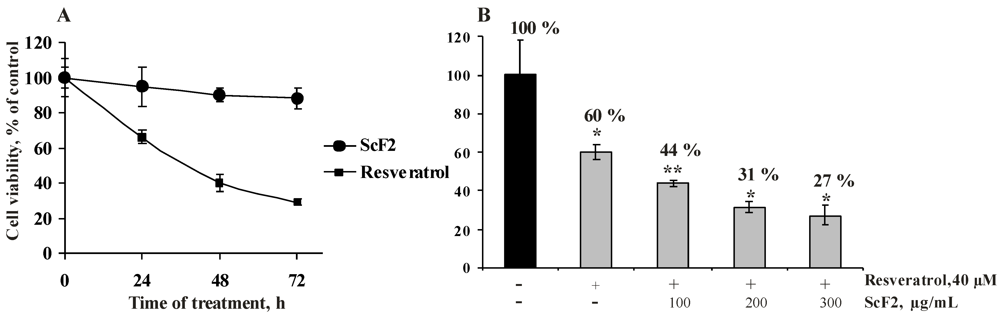
2.2.3. The Fucoidan from the Brown Alga Saccharina cichorioides Facilitates Resveratrol-Induced Apoptosis in Colon Cancer Cells
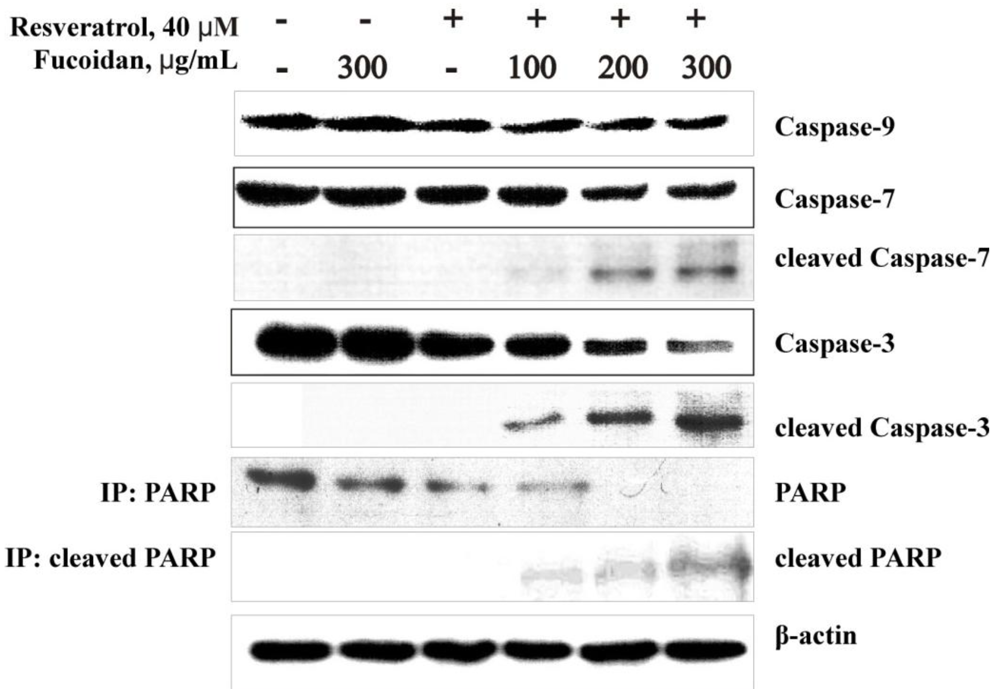

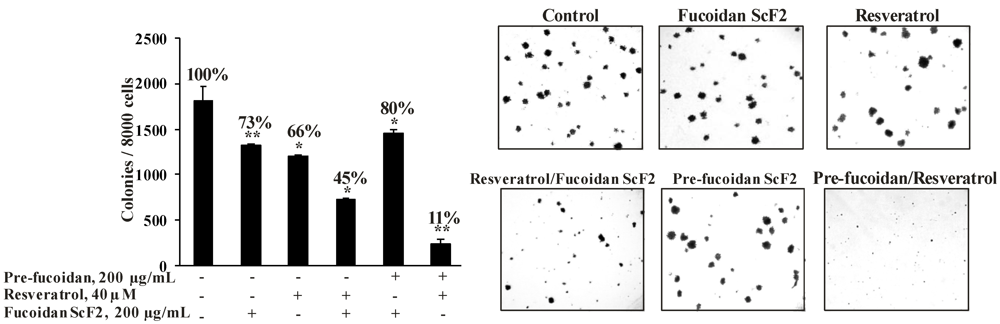
2.2.4. The Fucoidan from the Brown Alga Saccharina cichorioides Sensitizes Human Colon Cancer Cells to Resveratrol
3. Experimental Section
3.1. Chemicals and Cell Culture Reagents
3.2. Algal Material
3.3. Cell Lines
3.4. Isolation and Determination of the Fucoidan Structure
3.4.1. Extraction of Water-Soluble Polysaccharide from the Brown Alga S. cichorioides
3.4.2. Anion-Exchange Chromatography
3.4.3. Carbohydrate Content
3.4.4. Sulfate Group Content
3.4.5. Acid Hydrolysis
3.4.6. Analysis of Monosaccharide Composition
3.4.7. Desulfation of the Fucoidan
3.4.8. Determination of Molecular Weight
3.4.9. IR Spectroscopy
3.4.10. NMR Spectroscopy
3.5. Biological Assays
3.5.1. Cell Culture
3.5.2. Cytotoxicity Assay
3.5.3. Growth Inhibition Assay
3.5.4. Western Blotting Assay
3.5.5. Immunoprecipitation Assay
3.5.6. Flow Cytometry Assay
3.5.7. Soft Agar Clonogenic Assay
3.6. Statistical Analysis
4. Conclusion
Acknowledgements
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef]
- Chung, K.Y.; Saltz, L.B. Adjuvant therapy of colon cancer: Current status and future directions. Cancer J. 2007, 13, 192–197. [Google Scholar]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Hengartner, M.O. Programmed cell death: Alive and well in the new millennium. Trends Cell Biol. 2001, 11, 526–534. [Google Scholar] [CrossRef]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar]
- Macdonald, J.S.; Astrow, A.B. Adjuvant therapy of colon cancer. Semin. Oncol. 2001, 28, 30–40. [Google Scholar] [CrossRef]
- Aggarwal, B.; Bhardwaj, A.; Aggarwal, R.; Seeram, N.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Matic, I.; Zizak, Z.; Simonovic, M.; Simonovic, B.; Godevac, D.; Savikin, K.; Juranic, Z. Cytotoxic effect of wine polyphenolic extracts and resveratrol against human carcinoma cells and normal peripheral blood mononuclear cells. J. Med. Food 2010, 13, 851–862. [Google Scholar] [CrossRef]
- Roberti, M.; Pizzirani, D.; Simoni, D.; Rondanin, R.; Baruchello, R.; Bonora, C.; Buscemi, F.; Grimaudo, S.; Tolomeo, M. Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J. Med. Chem. 2003, 46, 3546–3554. [Google Scholar] [CrossRef]
- Minutolo, F.; Sala, G.; Bagnacani, A.; Bertini, S.; Carboni, I.; Placanica, G.; Prota, G.; Rapposelli, S.; Sacchi, N.; Macchia, M.; et al. Synthesis of a resveratrol analogue with high ceramide-mediated proapoptotic activity on human breast cancer cells. J. Med. Chem. 2005, 48, 6783–6786. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar]
- Cree, I.; Knight, L.; Di Nicolantonio, F.; Sharma, S.; Gulliford, T. Chemosensitization of solid tumor cells by alteration of their susceptibility to apoptosis. Curr. Opin. Invest. Drugs 2002, 3, 641–647. [Google Scholar]
- Wijesekara, I.; Pangestuti, R.; Kim, S. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS One 2011, 6, e27441. [Google Scholar]
- Rocha, H.A.; Franco, C.R.; Trindade, E.S.; Veiga, S.S.; Leite, E.L.; Nader, H.B.; Dietrich, C.P. Fucan inhibits Chinese hamster ovary cell (CHO) adhesion to fibronectin by binding to the extracellular matrix. Planta Med. 2005, 71, 628–633. [Google Scholar] [CrossRef]
- Ye, J.; Li, Y.; Teruya, K.; Katakura, Y.; Ichikawa, A.; Eto, H.; Hosoi, M.; Nishimoto, S.; Shirahata, S. Enzyme-digested fucoidan extracts derived from seaweed Mozuku of Cladosiphon novae-caledoniae kylin inhibit invasion and angiogenesis of tumor cells. Cytotechnology 2005, 47, 117–126. [Google Scholar]
- Vishchuk, O.S.; Ermakova, S.P.; Pham, T.D.; Ly, B.M.; Zvyagintseva, T.N. Antitumor activity of fucoidans from brown algae. J. Pac. Med. 2009, 3, 92–96. [Google Scholar]
- Anastyuk, S.D.; Shevchenko, N.M.; Nazarenko, E.L.; Imbs, T.I.; Gorbach, V.I.; Dmitrenok, P.S.; Zvyagintseva, T.N. Structural analysis of a highly sulfated fucan from the brown alga Laminaria cichorioides by tandem MALDI and ESI mass spectrometry. Carbohydr. Res. 2010, 345, 2206–2212. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.; Preobrazhenskaya, M.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar]
- Shevchenko, N.M.; Anastyuk, S.D.; Gerasimenko, N.I.; Dmitrenok, P.S.; Isakov, V.V.; Zvyagintseva, T.N. Polysaccharide and lipid composition of the brown seaweed Laminaria gurjanovae. Bioorg. Chim. 2007, 33, 96–107. [Google Scholar]
- Synytsya, A.; Kim, W.; Kim, S.; Pohl, R.; Synytsya, A. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Yang, C.; Chung, D.; Shin, I.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar]
- Lee, N.Y.; Ermakova, S.P.; Zvyagintseva, T.N.; Kang, K.W.; Dong, Z.; Choi, H.S. Inhibitory effects of fucoidan on activation of epidermal growth factor receptor and cell transformation in JB6 Cl41 cells. Food Chem. Toxicol. 2008, 46, 1793–1800. [Google Scholar]
- Fukahori, S.; Yano, H.; Akiba, J.; Ogasawara, S.; Momosaki, S.; Sanada, S.; Kuratomi, K.; Ishizaki, Y.; Moriya, F.; Yagi, M.; Kojiro, M. Fucoidan, a major component of brown seaweeds, prohibits the growth of human cancer cells in vitro. Mol. Med. Rep. 2008, 1, 537–542. [Google Scholar]
- Mahyar-Roemer, M.; Katsen, A.; Mestres, P.; Roemer, K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int. J. Cancer. 2001, 94, 615–622. [Google Scholar] [CrossRef]
- Chang, S.; Huang, S. Experimental study on effect of compounds in Inhibiting HCT-116 human colon cancer cells: The preliminary results. J. Soc. Colon Rectal Surgeon. 2010, 21, 121–129. [Google Scholar]
- Haneji, K.; Matsuda, T.; Tomita, M.; Kawakami, H.; Ohshiro, K.; Uchihara, J.; Masuda, M.; Takasu, N.; Tanaka, Y.; Ohta, T.; et al. Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr. Cancer 2005, 52, 189–201. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Jin, Z.; El-Deiry, W. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K. Resveratrol modulation of signal transduction in apoptosis and cell survival: A mini-review. Cancer Detect. Prev. 2006, 30, 217–223. [Google Scholar] [CrossRef]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Boo, H.J.; Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, S.Y.; Cho, H.; Yoo, E.S.; Kang, H.K. Fucoidan from Undaria pinnatifida induces apoptosis in A549 human lung carcinoma cells. Phytother. Res. 2011, 25, 1082–1086. [Google Scholar] [CrossRef]
- Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Boo, H.J.; Kwon, J.M.; Koh, Y.S.; Hyun, J.W.; Park, D.B.; Yoo, E.S.; et al. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1760–1764. [Google Scholar] [CrossRef]
- Nishino, T.; Nishioka, C.; Ura, H.; Nagumo, T. Isolation and partial characterization of a novel amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohydr. Res. 1994, 255, 213–224. [Google Scholar] [CrossRef]
- Imbs, T.I.; Shevchenko, N.M.; Sukhoverkhov, S.V.; Semenova, T.L.; Skriptsova, A.V.; Zvyagintseva, T.N. Seasonal variations of the composition and structural characterisitcs of polysaccharides from the brown alga Costaria costata. Chem. Nat. Comp. 2009, 45, 786–791. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Ermakova, S.; Sokolova, R.; Kim, S.; Um, B.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef]
- Huang, C.; Ma, W.; Goranson, A.; Dong, Z. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis 1999, 20, 237–242. [Google Scholar] [CrossRef]
- She, Q.B.; Ma, W.Y.; Wang, M.; Kaji, A.; Ho, C.T.; Dong, Z. Inhibition of cell transformation by resveratrol and its derivatives: Differential effects and mechanisms involved. Oncogene 2003, 22, 2143–2150. [Google Scholar] [CrossRef]
- Philchenkov, A.; Zavelevich, M.; Imbs, T.; Zvyagintseva, T.; Zaporozhets, T. Sensitization of human malignant lymphoid cells to etoposide by fucoidan, a brown seaweed polysaccharide. Exp. Oncol. 2007, 29, 181–185. [Google Scholar]
- Shevchenko, N.M.; Imbs, T.I.; Urvantseva, A.I.; Kusaykin, M.I.; Kornienko, V.G.; Zvyagintseva, T.N.; Elyakova, L.A. Method of processing seaweed. Patent WO 2005/014657, 17 February 2005. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar]
- Waffenschmidt, S.; Jaenicke, L. Assay of reducing sugars in the nanomole range with 2,2′-bicinchoninate. Anal. Biochem. 1987, 165, 337–340. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Colburn, N.H.; Wendel, E.J.; Abruzzo, G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: Mitogen-resistant variants are sensitive to promotion. Proc. Natl. Acad. Sci. USA 1981, 78, 6912–6916. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The Effect of Sulfated (1→3)-α-l-Fucan from the Brown Alga Saccharina cichorioides Miyabe on Resveratrol-Induced Apoptosis in Colon Carcinoma Cells. Mar. Drugs 2013, 11, 194-212. https://doi.org/10.3390/md11010194
Vishchuk OS, Ermakova SP, Zvyagintseva TN. The Effect of Sulfated (1→3)-α-l-Fucan from the Brown Alga Saccharina cichorioides Miyabe on Resveratrol-Induced Apoptosis in Colon Carcinoma Cells. Marine Drugs. 2013; 11(1):194-212. https://doi.org/10.3390/md11010194
Chicago/Turabian StyleVishchuk, Olesia S., Svetlana P. Ermakova, and Tatyana N. Zvyagintseva. 2013. "The Effect of Sulfated (1→3)-α-l-Fucan from the Brown Alga Saccharina cichorioides Miyabe on Resveratrol-Induced Apoptosis in Colon Carcinoma Cells" Marine Drugs 11, no. 1: 194-212. https://doi.org/10.3390/md11010194




