The Red Seaweed Gracilaria gracilis as a Multi Products Source
Abstract
:1. Introduction
2. Results and Discussion
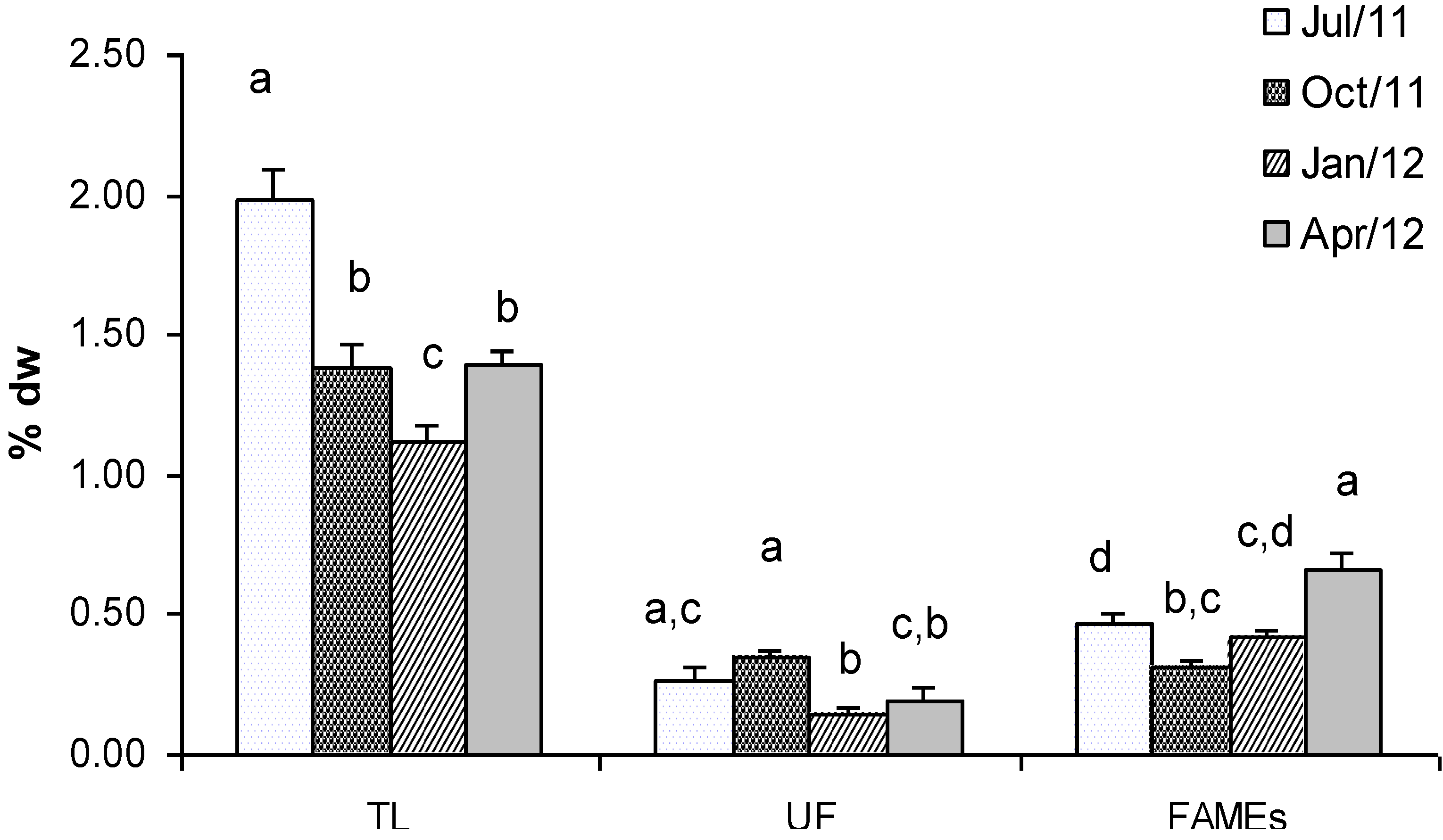
2.1. Lipids and Fatty Acids (FAMEs)
| Fatty Acids Methyl Esters | Structure | Fatty Acid Content (%) | |||
|---|---|---|---|---|---|
| 11 July | 11 October | 12 January | 12 April | ||
| Methyl decanoate | C10:0 | 0.05 a | 0.03 b | nd | 0.03 b |
| Methyl undecanoate | C11:0 | 0.01 | nd | nd | nd |
| Methyl dodecanoate | C12:0 | 0.28 a | 0.35 a | 0.06 b | 0.12 c |
| Methyl tridecanoate | C13:0 | 0.06 a | 0.07 a | 0.02 b | 0.03 b |
| Methyl 9-tetradecenoate | 9-C14:1 | 0.02 a | 0.39 b | 0.74 c | 0.55 d |
| Methyl tetradecanoate | C14:0 | 5.48 a | 5.55 a | 3.48 b | 5.13 a |
| cis-10 Pentadecenoic Acid Methyl Ester | 10-C15:1 | nd | 0.15 a | 0.03 b | 0.04 b |
| Pentadecanoic Acid Methyl Ester | C15:0 | 1.03 a | 1.32 b | 0.52 c | 0.53 c |
| Palmitoleic Acid Methyl Ester | 9-C16:1 | 3.84 a | 8.19 b | 2.21 c | 8.81 d |
| Palmitic Acid Methyl Ester | C16:0 | 25.67 a | 38.13 b | 28.55 c | 29.29 c |
| cis-10 Heptadecenoic Acid Methyl Ester | 10-C17:1 | 0.14 a | 1.12 b | 0.23 c | 0.24 c |
| Heptadecanoic Acid Methyl Ester | C17:0 | 0.64 a | 1.14 b | 0.20 c | 0.30 c |
| Linolenic Acid Methyl Ester | C18:3n-3 | 0.42 a | 0.23 b | 0.09 c | 3.88 d |
| Linoleic Acid Methyl Ester | C18:2n-6c | 4.85 a | 2.49 b | 1.33 c | 4.14 d |
| Oleic Acid Methyl Ester | C18:1n-9c | 10.78 a | 8.79 b | 5.76 c | 9.12 b |
| Elaidic Acid Methyl Ester | C18:1n-9t | 4.16 a | 6.15 b | 3.22 c | 2.02 d |
| Stearic Acid Methyl Ester | C18:0 | 3.35 a | 3.62 a | 1.87 b | 2.30 c |
| Arachidonic Acid Methyl Ester | C20:4n-6 | 33.27 a | 16.05 b | 47.78 c | 38.30 d |
| cis-5-8-11-14-17-Eicosapentaenoic Acid Methyl Ester | C20:5n-3 | 1.13 a | 1.84 b | 0.24 c | 3.93 d |
| cis-11,14,17-Eicosatrienoic Acid Methyl Ester | C20:3n-3 | 2.48 a | 1.23 b | 2.07 c | 2.82 a |
| cis-11,14-Eicosdienoic Acid Methyl Ester | 11,14-C20:2 | 0.32 a | 0.25 b | 0.23 b,c | 0.19 c |
| cis-8,11,14-Eicosatrienoic Acid Methyl Ester | 8,11,14-C20:3n-6 | nd | 0.32 a | 0.71 b | nd |
| cis-11 Eicosenoic Acid Methyl Ester | 11-C20:1 | 0.28 | nd | nd | nd |
| Arachidic Acid Methyl Ester | C20:0 | 0.15 a | 0.40 b | 0.08 c | 0.61 d |
| Heneicosanoic Acid Methyl Ester | C21:1 | 0.02 a | 0.02 a | 0.04 b | 0.02 a |
| cis-4,7,10,13,16,19-Docosahexaenoic Acid Methyl Ester | C22:6n-3 | 0.23 a | 0.43 b | 0.03 c | 0.12 d |
| Erucidic Acid Methyl Ester | C22:1n-9 | 0.65 a | 0.34 b | 0.19 c | 0.13 c |
| Docosanoic Acid Methyl Ester | C22:0 | 0.26 a | 0.38 b | 0.09 c | 0.19 d |
| Tricosanoic Acid Methyl Ester | C23:0 | 0.08 a | 0.16 b | 0.02 c | 0.02 c |
| cis-15-Tetracosenoic Acid Methyl Ester | 15-C24:1 | 0.09 a | 0.31 b | 0.15 c | 0.15 c |
| Tetracosanoic Acid Methyl Ester | C24:0 | 0.27 a | 0.56 b | 0.05 c | 0.15 d |
| TOTAL (mg g−1 dry weight) | 4.71 a | 3.14 b | 4.18 a | 6.67 c | |
| SFA (% w/w) | 37.33 a | 51.71 b | 34.94 c | 34.19 c | |
| MUFA (% w/w) | 19.98 a | 25.31 b | 12.54 c | 18.60 a | |
| PUFA (% w/w) | 42.70 a | 22.52 b | 51.77 c | 47.18 a | |
| PUFAω6 (% w/w) | 38.12 a | 18.86 b | 49.82 c | 37.51 a | |
| PUFAω3 (% w/w) | 4.26 a | 3.73 a,b | 2.43 b | 9.51 c | |
| ω6/ω3 | 8.96 a | 5.06 b | 20.48 c | 3.95 b | |
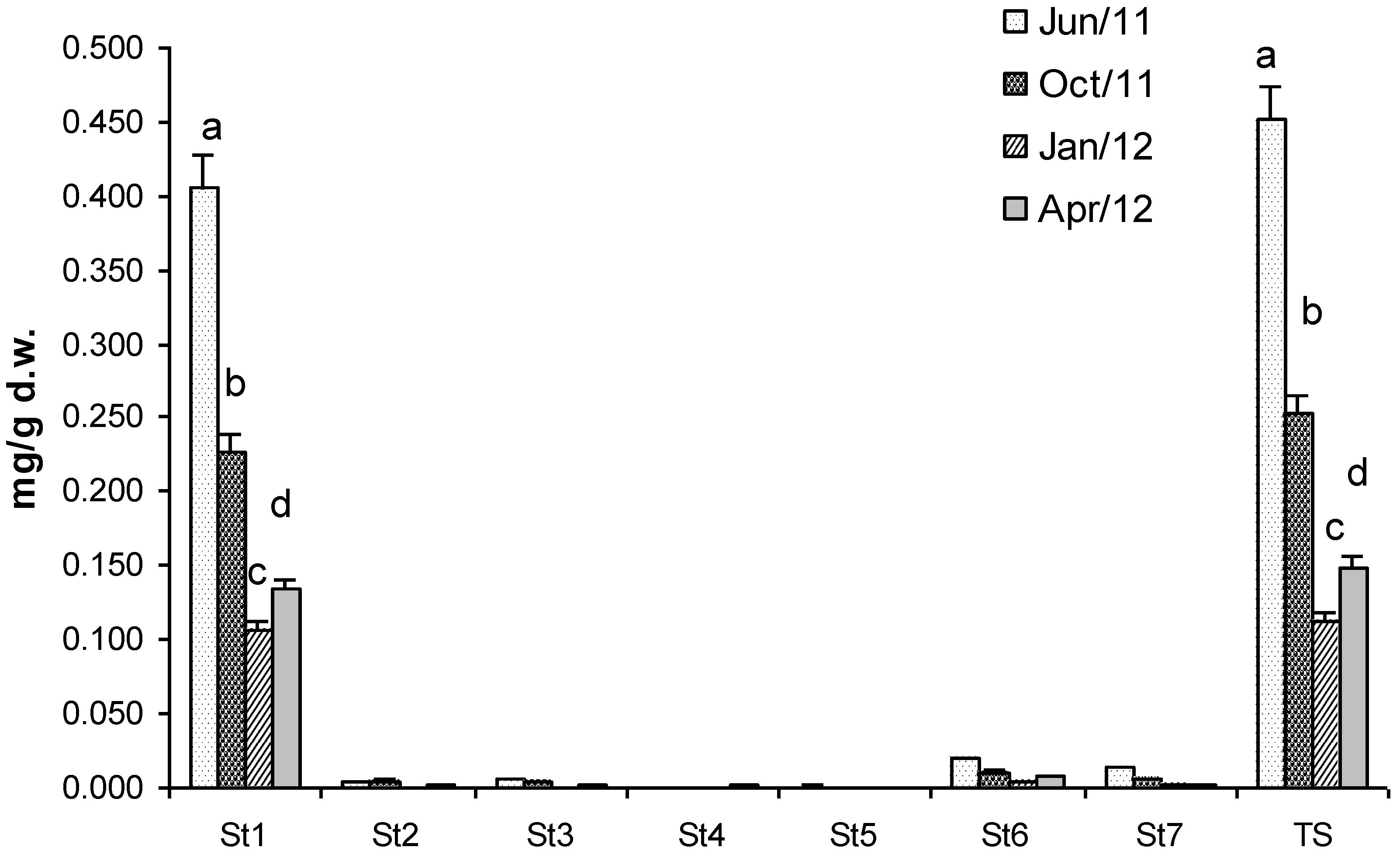
2.2. Sterols
2.3. Proteins
2.4. Phycobiliproteins
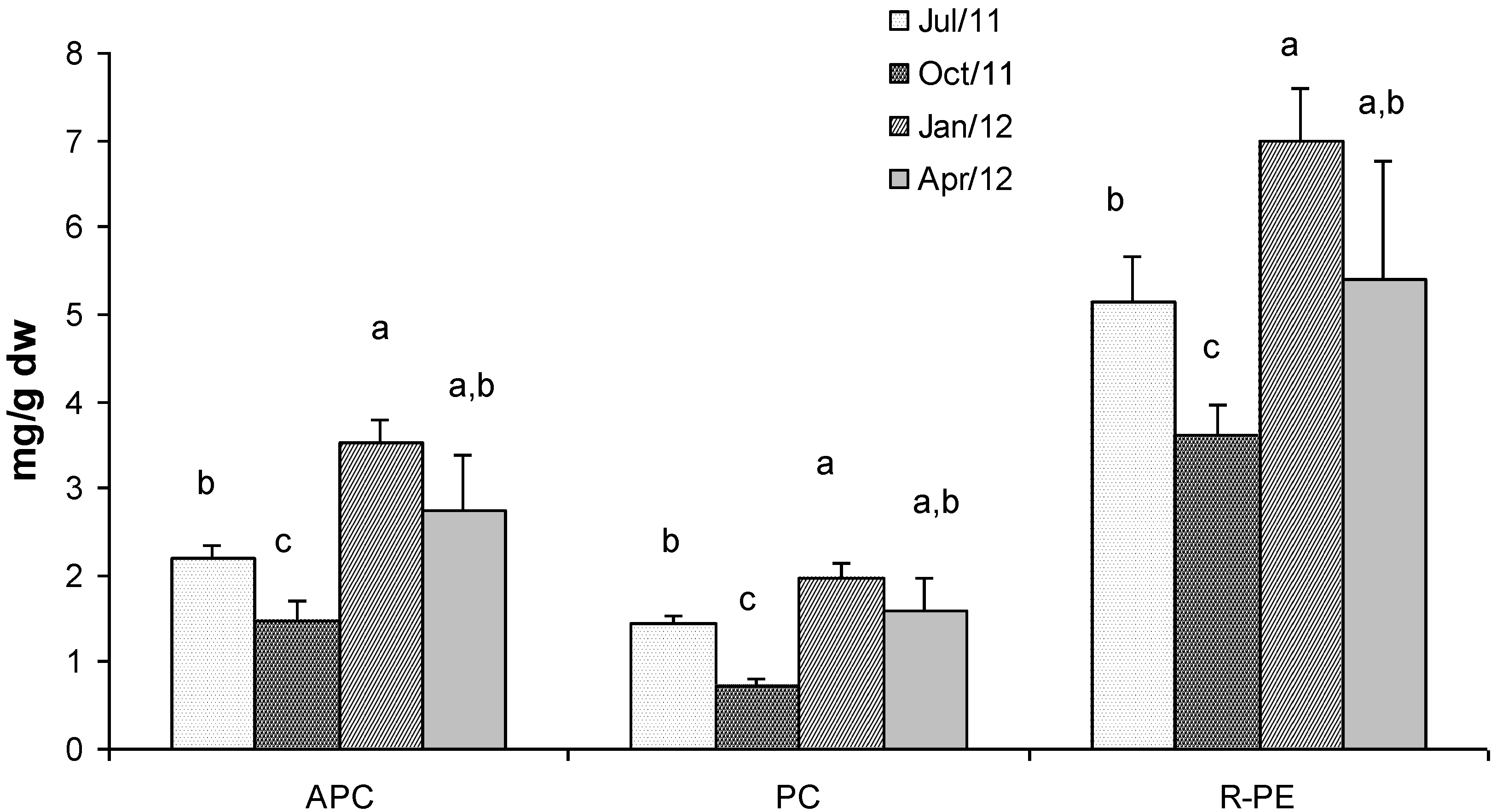
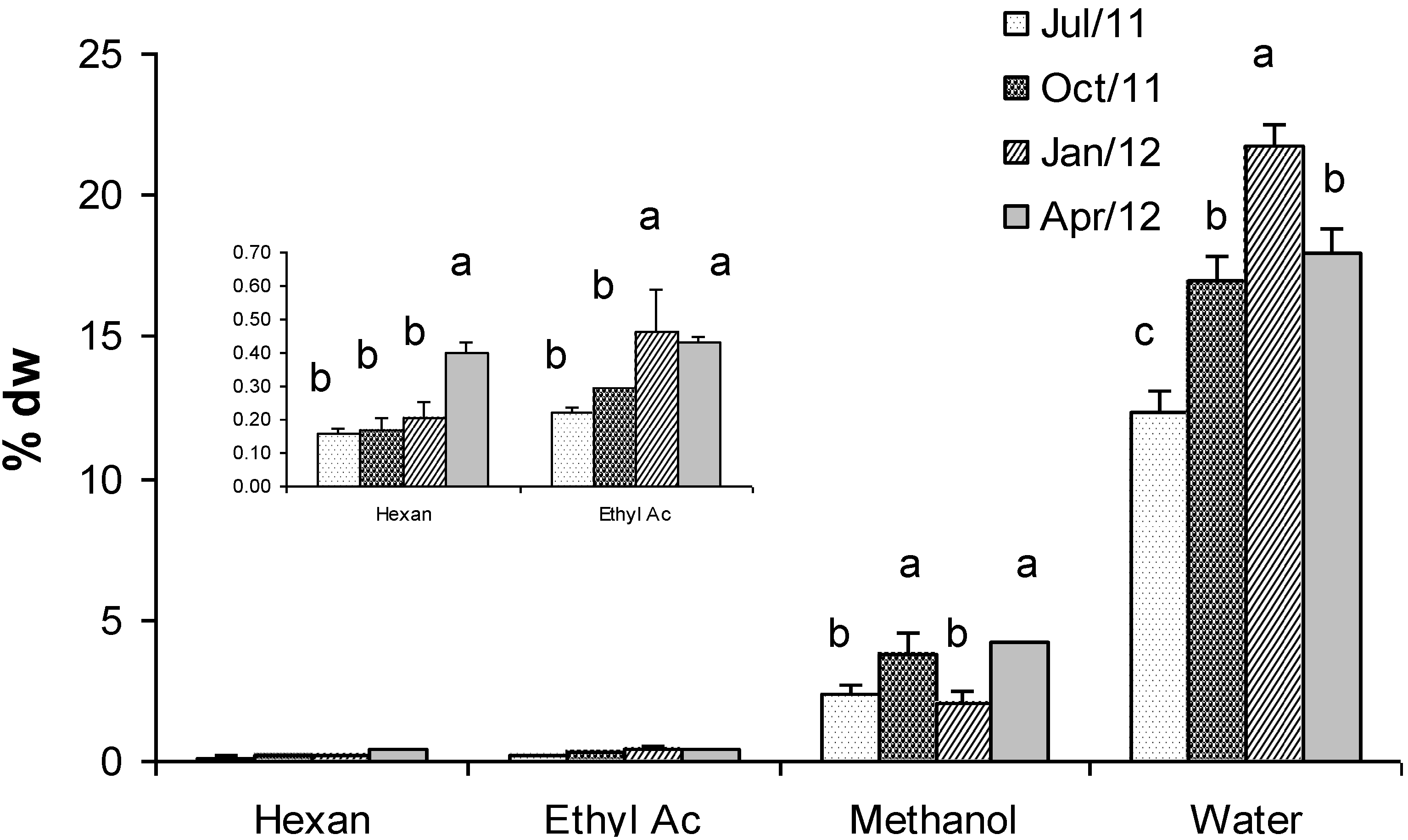
2.5. Fractionated Extraction of Algal Biomass for Antioxidant Assays and Analysis of Total Phenolic Content
2.6. Antioxidant Activity Assays
2.6.1. Ferric-Reducing Antioxidant Power (FRAP) Assay
| Extracts | |||||
|---|---|---|---|---|---|
| HE | EA | ME | WT | ||
| July/11 | 370.74 ± 18.5 a | 808.90 ± 40.4 a | 99.90 ± 5.0 a | 13.32 ± 0.7 a | 1917.93 ± 95.9 |
| October/11 | 114.28 ± 5.7 b | 312.47 ± 15.6 b | 26.90 ± 1.3 b | 9.06 ± 0.5 b | |
| January/12 | 189.23 ± 9.5 c | 62.02 ± 3.1 c | 53.05 ± 2.7 c | 9.19 ± 0.5 b | |
| April/12 | 117.24 ± 5.9 b | 29.82 ± 1.5 d | 50.14 ± 2.5 c | 14.61 ± 0.7 a | |
2.6.2. ABTS Assay
2.6.3. DPPH Assay
| Extracts | |||||
|---|---|---|---|---|---|
| HE | EA | ME | WT | ||
| July/11 | 0.09 ± 0.02 a | 0.43 ± 0.04 a | 0.06 ± 0.01 a | 0.07 ± 0.02 a | 0.28 ± 0.04 |
| October/11 | 0.07 ± 0.01 a | 0.18 ± 0.02 b | 0.02 ± 0.01 b | 0.05 ± 0.01 a | |
| January/12 | 0.16 ± 0.03 b | 0.26 ± 0.02 c | 0.06 ± 0.02 a | 0.10 ± 0.03 a,b | |
| April/12 | 0.26 ± 0.03 c | 0.34 ± 0.03 d | 0.03 ± 0.01 b | 0.15 ± 0.03 b | |
| Extracts | |||||
|---|---|---|---|---|---|
| Date | HE | EA | ME | WT | CTR (BHT) |
| 11 July | 1.1 ± 0.06 a | 0.82 ± 0.04 a | 2.94 ± 0.15 a | 10 ± 0.50 * | 0.67 ± 0.03 |
| 11 October | 3.43 ± 0.17 b | 2.55 ± 0.13 b | 5.73 ± 0.29 b | 30.4 ± 1.52 * | |
| 12 January | 3.32 ± 0.17 b | 3.47 ± 0.17 c | 9.72 ± 0.49 c | 33.17 ± 1.66 a | |
| 12 April | 4.29 ± 0.21 c | 7.03 ± 0.35 d | 8.72 ± 0.44 c | 35.03 ± 1.75 a | |
2.7. Total Phenolic Content
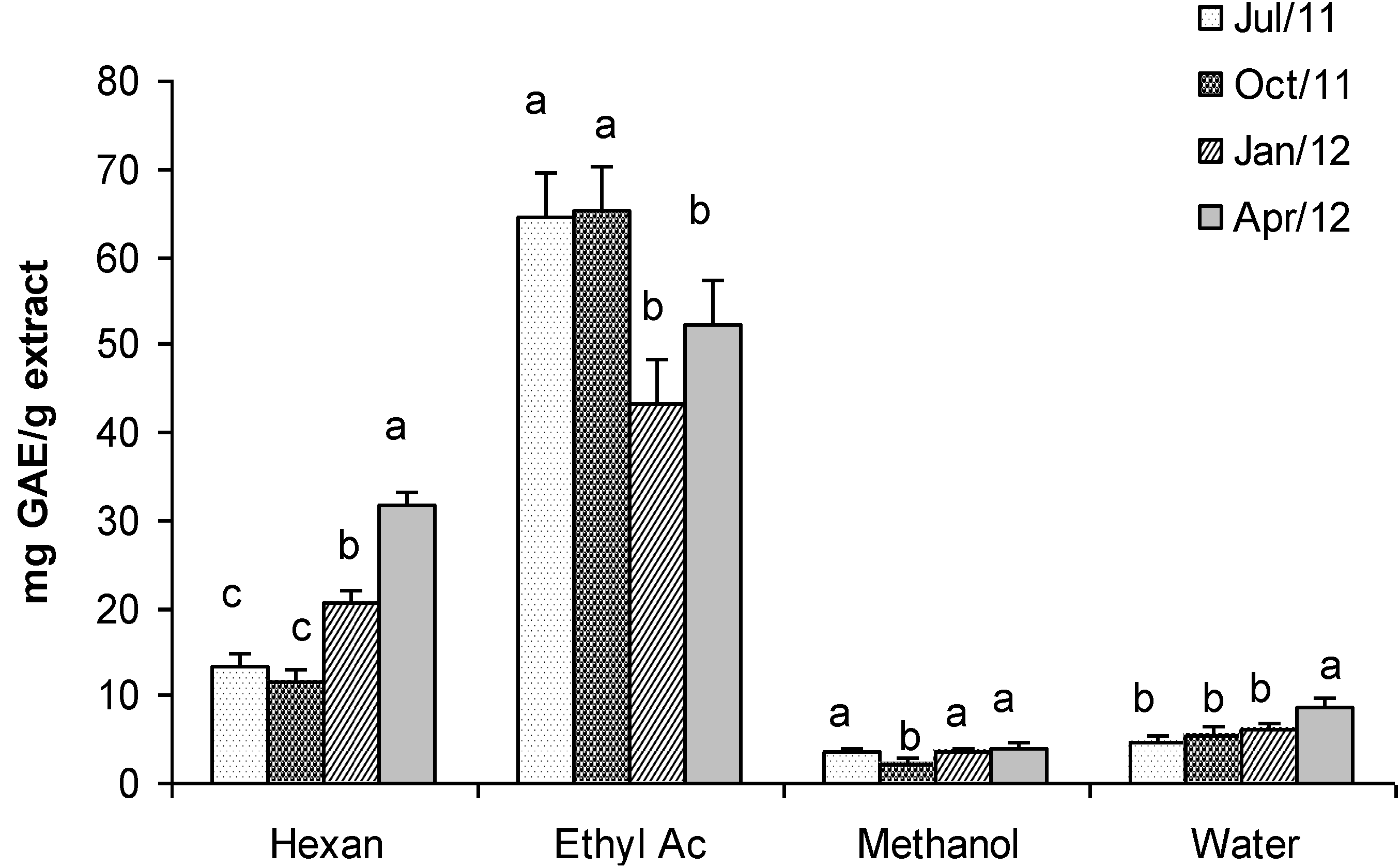
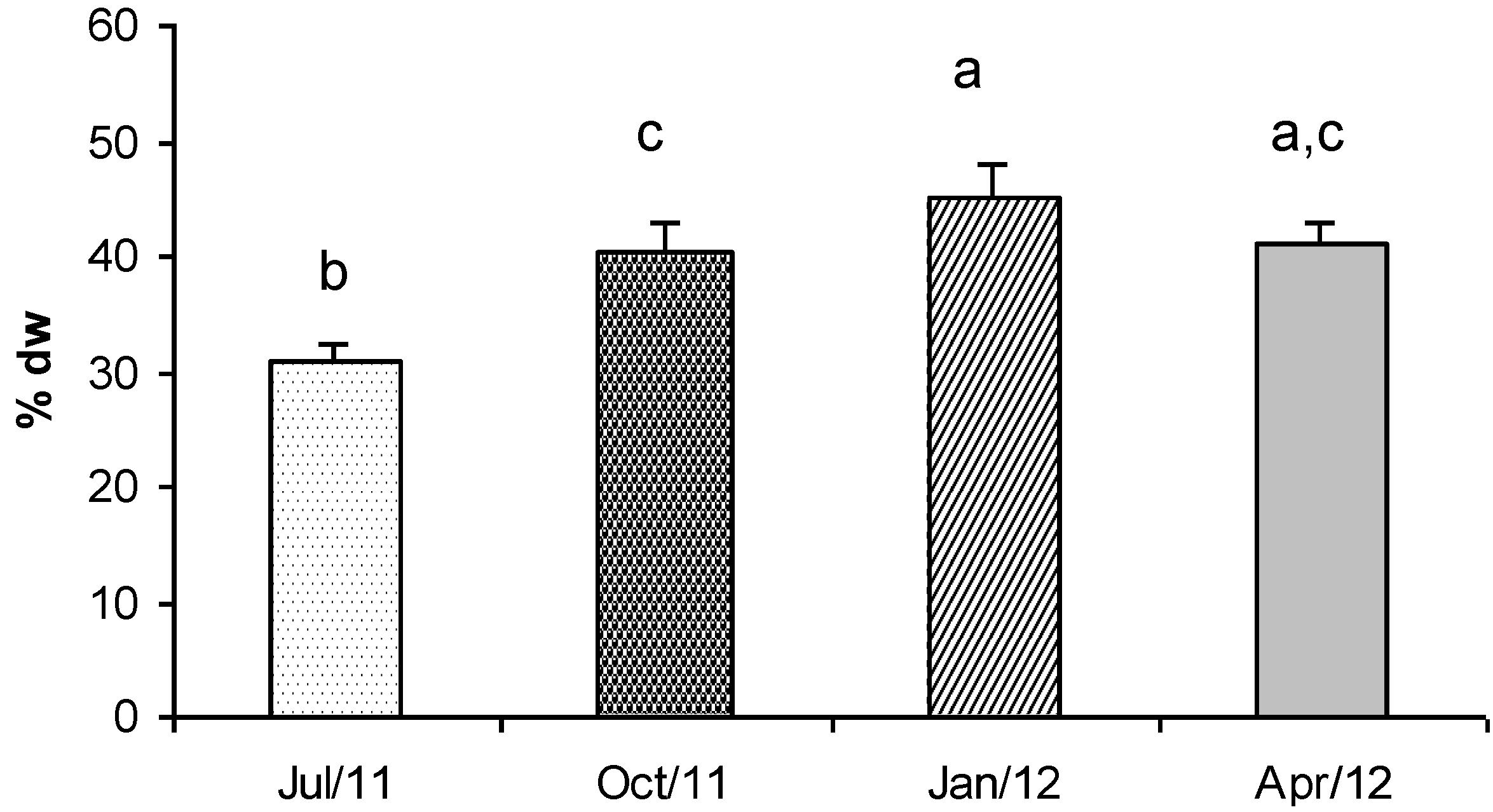
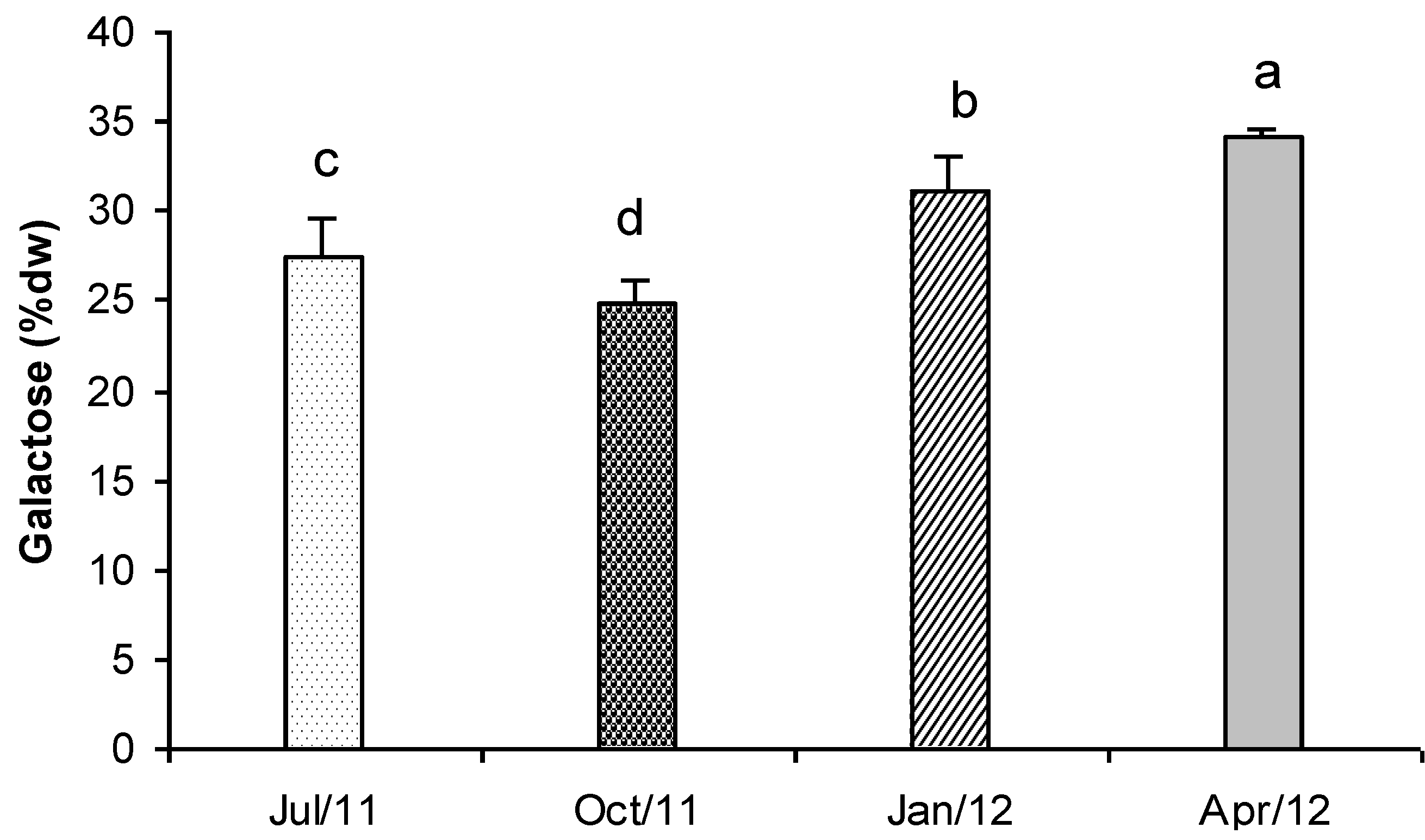
2.8. Total Carbohydrates
3. Experimental Section
3.1. Biomass Sampling
3.2. Lipid Extraction
3.3. Saponification of Lipids for Sterols Analysis
3.4. Sterols Analysis by Gaschromatography-Mass Spectrometry (GC-MS)
3.5. Fatty Acids Methyl Esters (FAMEs) Analysis
3.6. Protein Extraction and Analysis
3.7. Phycobiliprotein Extraction and Analysis
PC = 151.1A614 − 99.1A651
R-PE = 155.8A498.5 − 40.0A614 − 10.5A651
3.8. Fractionated Extraction of Algal Biomass
3.9. Antioxidant Activity Assays
3.9.1. Reducing Power: The FRAP Assay
3.9.2. ABTS Assay
3.9.3. DPPH Radical Scavenging Activity
3.10. Determination of Total Phenolic Content
3.11. Total Carbohydrates
3.12. Statistical Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Morrissey, M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar] [CrossRef]
- De Almeida, C.L.F.; de S. Falcão, H.; de M. Lima, G.R.; de A. Montenegro, C.; Lira, N.S.; de Athayde-Filho, P.F.; Rodrigues, L.C.; de Souza, M.F.V.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Year Book of Fishery and Aquaculture Statistics (2006); Food and Agricultural Organisation of the United Nations: Rome, Italy, 2008; Volumn 98, p. 57.
- Bourgougnon, N.; Stiger-Pouvreau, V. Red and Brown Macroalgae Along the French Coasts, Metropole and Overseas Departements and Territories. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology, 1st ed.; Se-Kwon, K., Ed.; JohnWiley & Sons, Ltd.: New Delhi, India, 2012; pp. 58–105. [Google Scholar]
- Ghosh, R.; Banerjee, K.; Mitra, A. Eco-Biochemical Studies of Common Seaweeds in the Lower Gangetic Delta. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology, 1st ed.; Se-Kwon, K., Ed.; JohnWiley & Sons, Ltd.: New Delhi, India, 2012; pp. 45–57. [Google Scholar]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Kadam, S.; Prabhasankar, P. Marine foods as functional ingredients in bakery and pasta products. Food Res. Int. 2010, 43, 1975–1980. [Google Scholar] [CrossRef]
- Capo, T.R.; Jaramillo, J.C.; Boyd, A.E.; Lapointe, B.E.; Serafy, J.E. Sustained high yields of Gracilaria (Rodophyta) grown in intensive large-scale culture. J. Appl. Phycol. 1999, 11, 143–147. [Google Scholar] [CrossRef]
- Kerton, F.M.; Liu, Y.; Omaria, K.W.; Hawboldt, K. Green chemistry and the ocean-based biorefinery. Green Chem. 2013, 15, 860–871. [Google Scholar] [CrossRef]
- Trotta, P. On the Rhodophyta Gracilaria confervoides Grev. in Lesina lagoon: Field Survey and in Vitro Culture. In Proceedings of the International Congress Phytodepuration and Biomass Utilization, Parma, Italy, 15–16 May 1981; pp. 91–96.
- D’Adamo, R.; Cecere, E.; Fabbrocini, A.; Petrocelli, A.; Sfriso, A. The Lagoons of Lesina and Varano. In Flora and Vegetation of the Italian Transitional Water Systems; Cecere, E., Petrocelli, A., Izzo, G., Sfriso, A., Eds.; Spinea, CORILA, Multigraph: Venezia, Italy, 2009; pp. 159–171. [Google Scholar]
- Trotta, P. La pesca, la qualità delle acque, gli scarichi in laguna. In Proceedings of the Conference La laguna di Lesina: Quali prospettive, Quali soluzioni, Lesina (FG), Italy, 11 December 1994; pp. 11–21.
- Manini, E.; Fiordelmondo, C.; Gambi, C.; Pusceddu, A.; Danovaro, R. Benthic microbial loop functioning in coastal lagoons: A comparative approach. Oceanol. Acta 2003, 26, 27–38. [Google Scholar] [CrossRef]
- Nonnis Marzano, C.; Scalera Liaci, L.; Franchini, A.; Gravina, F.; Mercurio, M.; Corriero, G. Distribution, persistence and change in the macrobenthos of the lagoon of Lesina (Apulia, southern Adriatic Sea). Oceanol. Acta 2003, 26, 57–66. [Google Scholar] [CrossRef]
- Francavilla, M. Riabilitazione delle acque lagunari salmastre attraverso la gestione di praterie di macroalghe di valore commerciale. Ph.D. Thesis, Università di Foggia, Facoltà di Agraria, Foggia, Italy, 2007. [Google Scholar]
- Francavilla, M.; Pineda, A.; Lin, C.S.K.; Franchi, M.; Trotta, P.; Romero, A.A.; Luque, R. Natural porous agar materials from macroalgae. Carbohydr. Polym. 2013, 92, 1555–1560. [Google Scholar]
- Budarin, V.L.; Zhao, Y.; Gronnow, M.J.; Shuttleworth, P.S.; Breeden, S.W.; Macquarrie, D.J.; Clark, J.H. Microwave-mediated pyrolysis of macro-algae. Green Chem. 2011, 13, 2330–2333. [Google Scholar]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar]
- Bernal, J.; Mendiola, J.A.; Ibañez, E.; Cifuentes, A. Advanced analysis of nutraceuticals. J. Pharm. Biomed. Anal. 2011, 55, 758–774. [Google Scholar]
- Burtin, P. Nutritional value of seaweeds. Electron. J. Environ. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- Mišurcová, L. Chemical Composition of Seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology, 1st ed.; Se-Kwon, K., Ed.; JohnWiley & Sons, Ltd.: New Delhi, India, 2012; pp. 181–182. [Google Scholar]
- Gressler, V.; Yokoya, N.Y.; Fujii, M.T.; Colepicolo, P.; Mancini Filho, J.; Pavan Torres, R.; Pinto, E. Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chem. 2010, 120, 585–590. [Google Scholar]
- Benatti, P.; Peluso, G.; Nicolai, R.; Calvani, M. Polyunsaturated fatty acids: Biochemical, nutritional and epigenetic properties. J. Am. Coll. Nutr. 2004, 23, 281–302. [Google Scholar]
- Von Schacky, C. The role of Omega-3 fatty acids in cardiovascular disease. Curr. Atheroscler. Rep. 2003, 5, 139–145. [Google Scholar]
- Maeda, H.; Tsukui, T.; Sashima, T.; Hosokawa, M.; Miyashita, K. Seaweed carotenoid, fucoxanthin, as multi-functional nutrient. Asia Pac. J. Clin. Nutr. 2008, 17, 196–199. [Google Scholar]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular diseases and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar]
- Fernandes, P.; Cabral, J.M.S. Phytosterols: Applications and recovery. Bioresour. Technol. 2007, 98, 2335–2350. [Google Scholar]
- Platt, D.; Pelled, D.; Shulman, A. Oils Enriched with Diacylglycerols and Phytosterolesters for Use in the Reduction of Cholesterol and Tryclycerides. PCT Patent WO2004069150, 23 September 2004. [Google Scholar]
- McDermid, K.J.; Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar]
- Marrion, O.; Fleurence, J.; Schwertz, A.; Guéant, J.-L.; Mamelouk, L.; Ksouri, J.; Villaume, C. Evaluation of protein in vitro digestibility of Palmaria palmata and Gracilaria verrucosa. J. Appl. Phycol. 2005, 17, 99–102. [Google Scholar]
- Renaud, S.M.; Luong-Van, J.T. Seasonal variation in the chemical composition of tropical Australian marine macroalgae. J. Appl. Phycol. 2006, 18, 381–387. [Google Scholar]
- Wen, X.; Peng, C.; Zhou, H.; Lin, Z.; Lin, G.; Chen, S.; Li, P. Nutritional composition and assessment of Gracilaria lemaneiformis Bory. J. Integr. Plant Biol. 2006, 48, 1047–1053. [Google Scholar]
- Marinho-Soriano, E.; Câmara, M.R.; Cabral, T.M.; Carneiro, M.A.A. Preliminary evaluation of the seaweed Gracilaria cervicornis (Rhodophyta) as a partial substitute for the industrial feeds used in shrimp (Litopenaeus vannamei) farming. Aquac. Res. 2007, 38, 182–187. [Google Scholar]
- Vernon, T.O.; Alexander, N.; Glazer, A.N.; Stryer, L. Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J. Cell Biol. 1982, 93, 981–986. [Google Scholar]
- Ayyagari, M.S.; Pande, R.; Kamtekar, S.; Marx, K.A.; Tripathy, S.K.; Gao, H.; Kumar, J.; Akkara, J.A.; Kaplan, D.L. Molecular assembly of proteins and conjugated polymers: Toward development of biosensors. Biotechnol. Bioeng. 1995, 45, 116–125. [Google Scholar]
- Glazer, A.N. Light harvesting by phycobilisome. Annu. Rev. Biophys. Biophys. Chem. 1985, 14, 47–77. [Google Scholar]
- Li, G.W.; Wang, G.C.; Li, Z.G.; Tseng, C.K. Biological effect of R-phycoerythrin-mediated photosensitization on DNA. Prog. Biochem. Biolophys. 2000, 27, 621–624. [Google Scholar]
- Niu, J.F.; Wang, G.C.; Tseng, C.K. Method for large-scale isolation and purification of R-phycoerythrin from red alga Polysiphonia urceolata Grev. Protein Expr. Purif. 2006, 49, 23–31. [Google Scholar]
- Bermejo, R.; Ruiz, E.; Acien, F.G. Recovery of B-phycoerythrin using expanded bed adsorption chromatography: Scale-up of the process. Enzym. Microb. Technol. 2007, 40, 927–933. [Google Scholar]
- Reaven, P.D.; Witzum, J.L. Oxidised LDL in atherogenesis. Role of dietary modification. Annu. Rev. Nutr. 1996, 16, 51–71. [Google Scholar]
- Aruoma, I.O. Antioxidant action of plant foods. Use of oxidative DNA damage, as a tool for studying antioxidant efficacy. Free Radic. Res. 1990, 30, 419–427. [Google Scholar]
- Shon, M.Y.; Kim, T.H.; Sung, N.J. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellius of Hymenochaetaceae) extracts. Food Chem. 2003, 82, 593–597. [Google Scholar]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant properties of methanol extracts of five Phillanthus species from India. Food Sci. Technol. 2007, 40, 344–352. [Google Scholar]
- Kuda, T.; Tsunekawa, M.; Goto, H.; Araki, Y. Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J. Food Compos. Anal. 2005, 18, 625–633. [Google Scholar]
- Duan, X.J.; Zhang, W.W.; Li, X.M.; Wang, B.G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006, 95, 37–43. [Google Scholar]
- Ganesan, P.; Chandini, S.K.; Kumar, C.S.; Bhaskar, N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour. Technol. 2008, 99, 2717–2723. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [Green Version]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmate) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar]
- Murugan, K.; Iyer, V.V. Antioxidant and Antiproliferative Activities of Marine Algae, Gracilaria edulis and Enteromorpha lingulata, from Chennai Coast. Int. J. Cancer Res. 2012, 8, 15–26. [Google Scholar]
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar]
- Prior, R.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar]
- Onofrejová, L.; Vašičková, J.; Klejdusa, B.; Stratil, P.; Mišurcovà, L.; Kràčmar, S.; Kopeckŷ, J.; Vaceka, J. Bioactive phenols in algae: The application of pressurized-liquid and solid-phase extraction techniques. J. Pharm. Biomed. Anal. 2010, 51, 464–470. [Google Scholar]
- Wijesekara, I.; Pangestuti, R.; Kima, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar]
- Marinho-Soriano, E.; Bourret, E. Effects of season on the yield and quality of agar from Gracilaria species (Gracilariaceae, Rhodophyta). Bioresour. Technol. 2003, 90, 329–333. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Francavilla, M.; Trotta, P.; Luque, R. Phytosterols from Dunaliella tertiolecta and Dunaliella salina: A potentially novel industrial application. Bioresour. Technol. 2010, 101, 4144–4150. [Google Scholar] [CrossRef]
- Von der Haar, T. Optimized protein extraction for quantitative proteomics of yeasts. PLoS One 2007, 2, e1078. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Kursar, T.A.; van der Meer, J.; Alberte, R.S. Light harvesting system of red alga Gracilaria tikvahiae. I. biochemical analyses of pigment mutations. Plant Physiol. 1983, 73, 353–360. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Faramarzi, M.A.; Mohammadi, N.; Soltani, N.; Oveisi, M.R.; Nafissi-Varcheh, N. Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J. Appl. Phycol. 2010, 22, 43–50. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma as a power: The FRAP assay. Anal. Biochem. 1976, 239, 70–76. [Google Scholar] [CrossRef]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Hu, C.C.; Lin, J.T.; Lu, F.J.; Chou, F.P.; Yang, D.J. Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 2008, 109, 439–446. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The Red Seaweed Gracilaria gracilis as a Multi Products Source. Mar. Drugs 2013, 11, 3754-3776. https://doi.org/10.3390/md11103754
Francavilla M, Franchi M, Monteleone M, Caroppo C. The Red Seaweed Gracilaria gracilis as a Multi Products Source. Marine Drugs. 2013; 11(10):3754-3776. https://doi.org/10.3390/md11103754
Chicago/Turabian StyleFrancavilla, Matteo, Massimo Franchi, Massimo Monteleone, and Carmela Caroppo. 2013. "The Red Seaweed Gracilaria gracilis as a Multi Products Source" Marine Drugs 11, no. 10: 3754-3776. https://doi.org/10.3390/md11103754