1. Introduction
During daily urban life, a causal relationship between the elevated urban air pollution and an increased severity of airway diseases, including lung fibrosis and lung cancers, has been supported by epidemiological and toxicological research [
1,
2]. Fibrosis, a result of chronic inflammatory reactions induced by a variety of stimuli, has recently garnered increasing attention [
3]. Fibrotic diseases such as idiopathic pulmonary fibrosis, liver cirrhosis, systemic sclerosis, progressive kidney disease, and cardiovascular fibrosis are threatening the public health [
3]. However, successful methods for treating fibrosis have been limited, and the lack of effective small-molecule medicines is one of the serious issues [
4,
5]. Therefore, the search for bioactive compounds from natural resources represents an emerging pharmacological and therapeutic area in the fight against excessive fibrotic diseases.
Benzopyran derivatives have been demonstrated to have considerable bioactivities, including anti-oxidative, anti-hypertensive, anti-microorganism, and anti-inflammatory properties [
6,
7,
8,
9]. In our previous studies, it was shown that xiamenmycin, a benzopyran compound with the structure of
N-((3,4-dihydro-3
S-hydroxy-2
S-methyl-2-(4′
R-methyl-3′
S-pentenyl)-2
H-1-benzopyran-6-yl)carbonyl)-threonine, was obtained by cultivating the mangrove-derived strain
Streptomyces xiamenensis 318, and it was found to have multiple biological effects toward inhibiting fibrosis,
i.e., the inhibition of excessive lung fibrosis
in vitro and the attenuation of hypertrophic scars by the suppression of local inflammation, and by the reduction of the effects of mechanical stress [
10,
11,
12]. The possible mechanism could be the inhibition of the mechanical stress-induced pro-fibrotic effects by suppressing proliferation, activation, and contraction of fibroblast, and inactivating focal adhesion kinase (FAK), p38, and Rho guanosine triphosphatase signaling [
10,
11,
12]. During excessive fibrogenesis, the existence of self-perpetuating loops for inflammation and extracellular matrix (ECM) accumulation, which results from the inflammatory response and the mechanical forces, is closely related to formation of fibrotic diseases [
13]. Xiamenmycin, aimed at the loops, has inhibitory effects on both inflammation and mechanotransduction; therefore, it may be a potential candidate for the development of new anti-fibrotic drugs. Thus, in our ongoing study, it is worthwhile to continue the chemical and pharmaceutical investigations of new benzopyran compounds.
Streptomyces are known as versatile producers of novel secondary metabolites from various biosynthetic pathways. The same species of
Streptomyces strains even if the strains originate from different ecological niches can be used to hunt for novel bioactive compounds. Not surprisingly, an affined strain of
S. xiamenensis 318, namely the strain M1-94P isolated from deep-sea sediments, can produce small amounts of potentially novel benzopyrans. As the secondary metabolic pathways in the microorganisms isolated from the deep sea normally remained dormant or weakly expressed under laboratory conditions, rational strain development was critical prior to extensive chemical investigation. Ribosomal engineering is a simple but practical approach for strain breeding by targeting the microbial ribosomal proteins or the subunits of RNA polymerase and has been widely used for strain improvement [
14,
15,
16,
17]. This strategy was therefore adopted to either increase the production of the benzopyran compound or stimulate the biosynthesis of novel benzopyrans by activating dormant or weakly expressed secondary metabolite biosynthetic genes in the strain
S. xiamenensis M1-94P.
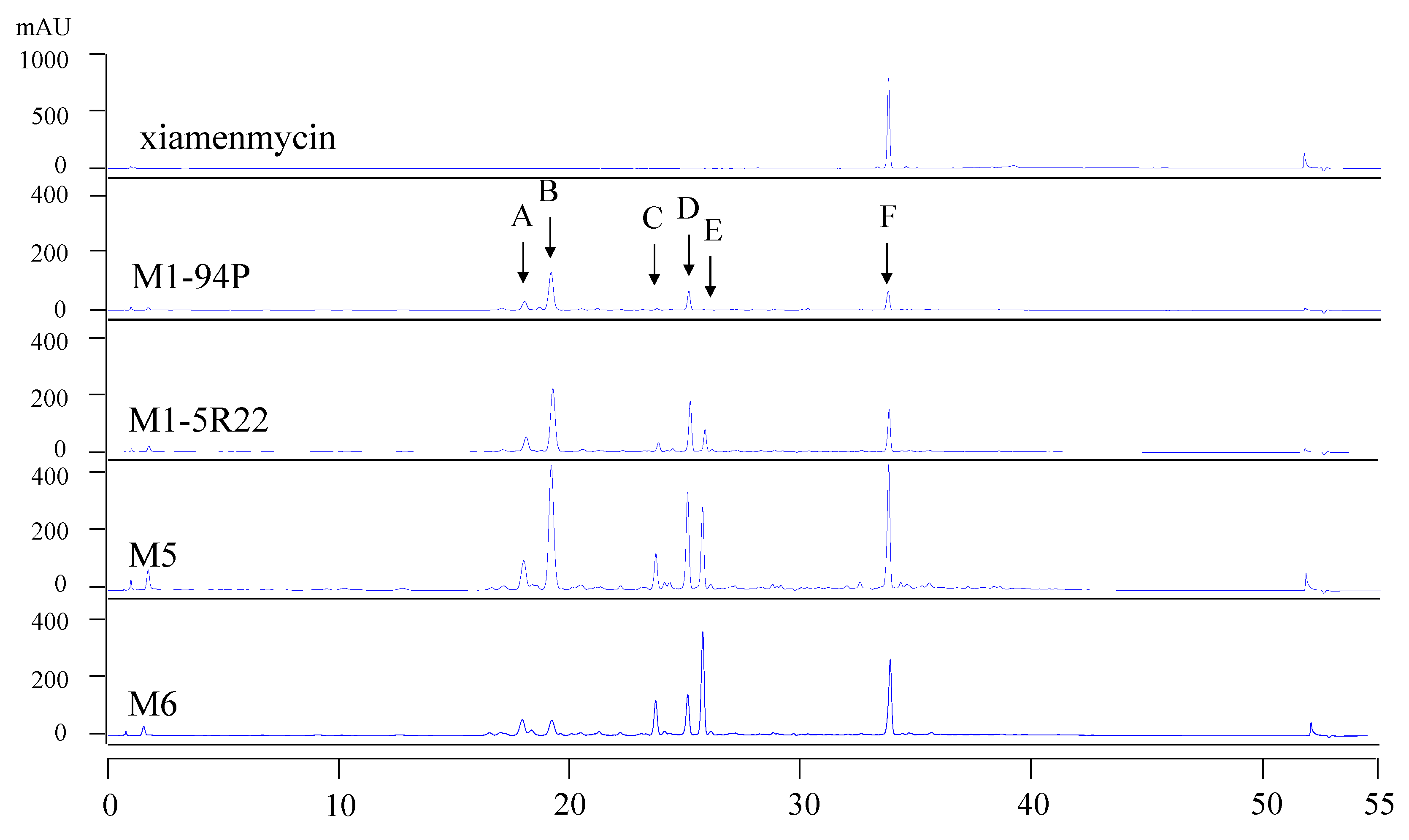
First, we introduced a spontaneous rifampicin resistant (Rif) mutation in strain M1-94P, and we then conferred streptomycin resistance (Str) to the Rif mutant by introducing a mutated rpsL gene. From the HPLC fingerprints of the constructed drug resistant mutants (M1-5R22, M5, and M6), the profiles of the metabolites were found to be altered, and the production of the benzopyran compounds improved. Two novel benzopyran compounds, xiamenmycin C (1) and D (2), were isolated from the crude extracts of M6, the strain with the highest productivity of benzopyran derivatives. The structures of 1 and 2 were identified through the analysis of extensive spectroscopic data. In this study, we showed that secondary metabolite production potential in the deep-sea derivatized Streptomyces strain S. xiamenensis M1-94P was awoken by ribosomal engineering. Our results also showed that 1 and 2 have anti-fibrotic activities, and 2 is even better than xiamenmycin, due to its lower concentration and higher inhibitory effect against WI26. Our work provided unique structures for use in the study of structure-bioactivity relationships, aimed at the development of anti-fibrotic compounds for the therapeutic treatment of excessive fibrotic diseases.
3. Discussion
The wild-type S. xiamenensis strain M1-94P, an affined strain of S. xiamenensis 318, was isolated from deep-sea sediments and used as a biosource in the search for new benzopyran compounds. The possibility of discovering novel chemical structures may be higher when shifting to an alien niche, such as deep-sea derived microbial strains, from the view of chemical ecology, but the production of secondary metabolites in the deep-sea derived microbes is normally quite low, due to physiological stress, when they are cultivated under the usual laboratory conditions. Therefore, the use of a rational strain development approach such as ribosomal engineering may be useful to increase the production of secondary metabolites in the laboratory.
Improving the production of the secondary metabolites by bacteria by modulating the ribosome components as well as other translating factors or RNAP [
15] has several advantages, including the ability to screen for drug-resistant mutations by simple selection on drug-containing plates and without prior genetic mutagenesis [
19]. It was reported that the introduction of multiple drug resistant mutations had the cumulative effect of increasing the production of secondary metabolites, which led to a dramatic increase in the production of the bioactive compounds. Rifampicin and streptomycin is a pair of drugs widely used in ribosomal engineering to screen potential high producers among drug-resistant mutants. In our case, the introduction of combined drug resistance to rifampicin and streptomycin in
S. xiamenensis M1-94P was a successful method to improve the production of benzopyran compounds for chemical isolation, although the detailed mechanism of the enhancement of the specific natural product compound remains unknown.
Because spontaneous rifampicin resistance mutants always have point mutations clustered in the so-called Rif domain on the
rpoB gene (encoding the β-subunit of RNA polymerase) and could mimic the ppGpp binding effect on the mutated RNAP [
20], we decided to screen for a Rif resistant mutant as the first step in the strain development. We have sequenced the
rpoB gene fragment of nine Rif mutants including six Rif mutants from 5 × MIC and 3 Rif mutants from 10 × MIC. All of the mutants have a point mutation in the Rif domain in the
rpoB gene.
There are two types of streptomycin resistance that could effectively alter ribosomal function. Determination of the mutation in the ribosomal RNA that engenders a low level of streptomycin resistance is not easy, but the introduction of a mutation into the highly conserved ribosomal protein S12 is conveniently achieved by site-directed mutagenesis using PCR (polymerase chain reaction). Furthermore, certain
rpsL mutations, resulting in the amino acid substitution of either K88E or L90K, that confer resistance to Str can increase the production of secondary metabolites in
Streptomyces [
21,
22,
23,
24,
25]. Thus, it is more rational to engineer ribosomal protein S12 as the second step in the strain development; thus, we introduced a mutated
rpsL gene to confer streptomycin resistance to the Rif mutant M1-5R22 and to interfere with its ribosomal function. As shown in the HPLC profiles, the production of the target compounds, xiamenmycin increased drastically in the Rif-Str double resistance strains M5 and M6.
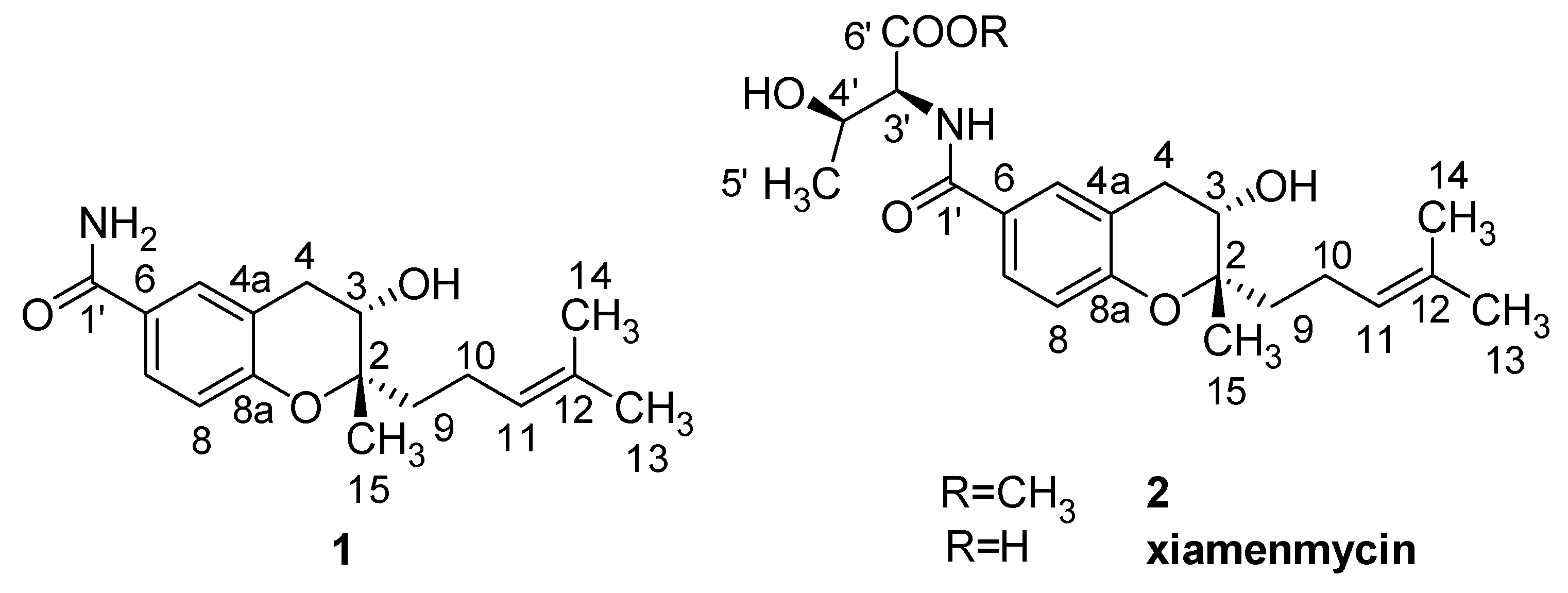
Xiamenmycin, xiamenmycin C (
1) and D (
2), with highly similar chemical structures, were all isolated from mutant M6 of deep-sea derived
S. xiamenensis M1-94P. Compared with xiamenmycin, obtained from our previous study [
11], xiamenmycin C had the same benzopyran skeleton and isoprene side chain. The only difference in the structures is in the amino acid moiety (on position 1′). Compound
1 is the possible precursor of xiamenmycin during biosynthesis. Compound
2 is methyl ester of xiamenmycin, which may be synthesized in the tailing step of the biosynthetic pathway. From the view of combinatorial biosynthesis, more “unnatural” natural products may be produced by the elucidation of the biosynthetic pathway for the synthesis of benzopyran compounds.
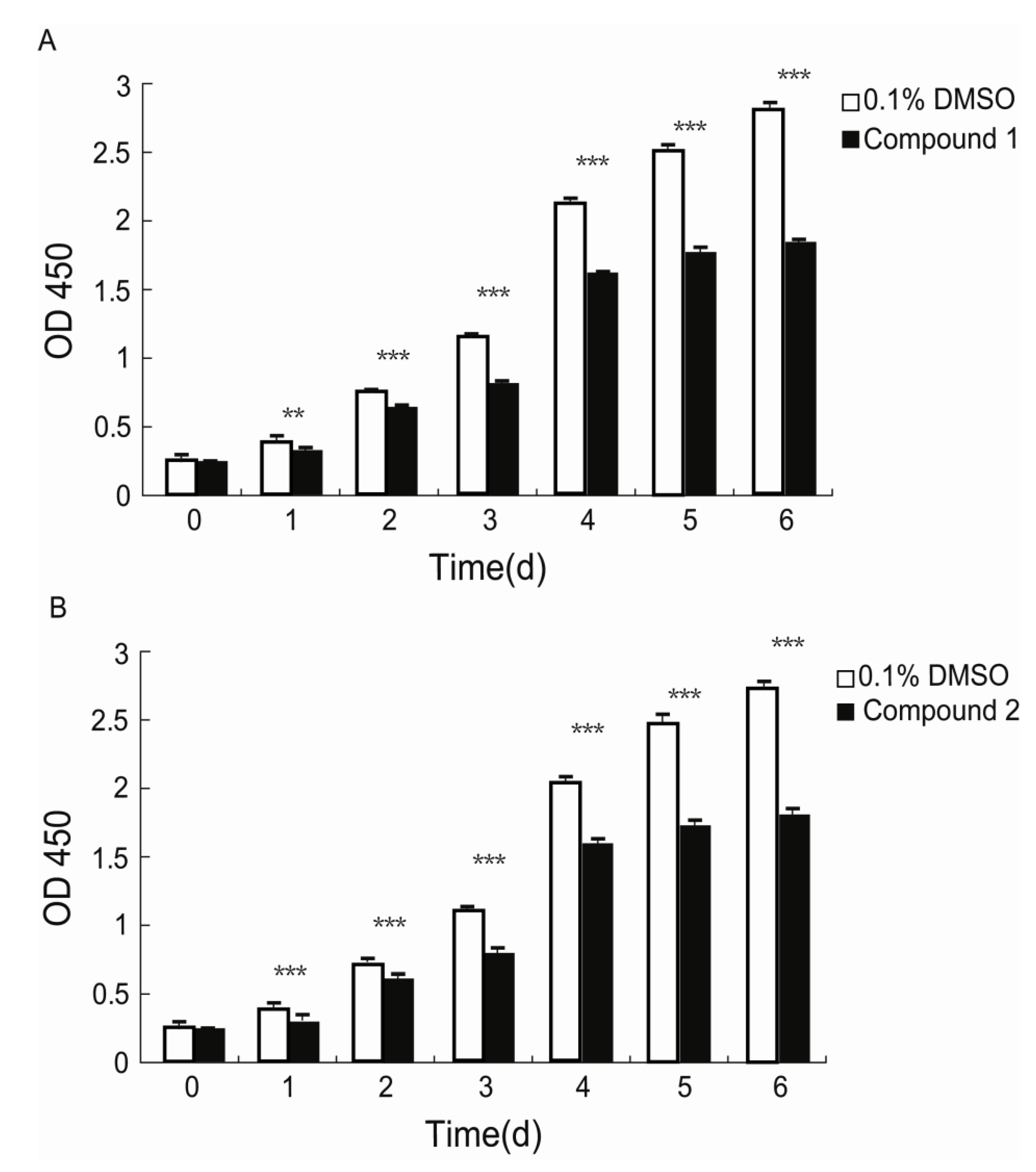
Xiamenmycin C (
1) was found to exhibit better inhibitory effects on the cell proliferation of human lung fibroblasts (WI26) using lower doses compared with xiamenmycin. It seems that the benzopyran skeleton is crucial for the anti-fibrotic activity. Xiamenmycin is an antifibrotic small molecule that targets the inflammatory and mechanical stress responses, the two pivotal pathological processes that occur during excessive fibrogenesis [
12]. Xiamenmycin D (
2) showed similar anti-fibrotic activity, compared with xiamenmycin. It indicated the methylation on position 6′ may not affect the bioactivity significantly. As a type of potential anti-fibrotic drug, more diverse benzopyran structures are needed for the investigation of the structure-activity relationship (SAR). For example, compounds with distinct substituent groups on the benzopyran skeleton are expected from natural sources, or a variation in the benzopyran configuration could be achieved by total or partial synthesis.
In summary, our results showed that xiamenmycin C and D, two new benzopyran structures, can be isolated from the deep-sea derived S. xiamenensis M1-94P. Ribosomal engineering, i.e., the introduction of a spontaneous rifampicin resistance mutation combined with the introduction of a streptomycin resistant mutation, was performed to generate mutant M6 with enhanced secondary metabolite production. As a promising candidate for treating excessive fibrotic diseases, the effects of xiamenmycin and its derivatives on mechanical stress and inflammation in association with various fibroblast cellular behaviors will be examined in the next stage of research.
4. Experimental Section
4.1. General
1H and 13C NMR spectra were recorded with Bruker DRX-500 and Advance Ⅲ-600 NMR spectrometers using the solvent as an internal standard (DMSO-d6 δ = 2.51 and 40.0 ppm, respectively). Coupling constants (J) are reported in Hertz (Hz) and chemical shifts (δ) are expressed in parts per million (ppm). Optical rotation was measured by a JASCO P-2000 polarimeter, and CD spectra were recorded on a J-815 spectropolarimeter (JASCO, Gross-Umstadt, Germany) at room temperature. UPLC-HRMS was measured on a Waters ACQUITY UPLC system equipped with a binary solvent delivery manager and a sample manager coupled with a Waters Micromass Q-TOF Premier Mass Spectrometer equipped with an electrospray interface (Waters Corporation, Milford, MA, USA). HPLC was performed on Agilent Technologies 1200 series instrument (Agilent Technologies, Wadbronn, Germany). Column chromatography was carried out with Sephadex LH-20 (40–70 μm, Amersham Pharmacia Biotech AB, Uppsala, Sweden), silica gel (200–300 mesh, Qingdao Marine Chemical, Inc., Qingdao, China), Lichroprep reversed-phase RP-18 silica gel (40–63 μm, Merck, Darmstadt, Germany) and silica gel H (10–40 μm, Qingdao, China). Analytical HPLC was carried out on an Agilent XDB-C18 column (4.6 × 150 mm, 5 μm) with a flow rate of 1 mL/min. Organic solvents for HPLC were analytical grade and were purchased from Merck KGaA (Darmstadt, Germany).
4.2. The Original Strain Materials
The Streptomyces xiamenensis strain M1-94P was isolated from a deep-sea sediment sample collected at the depth of 2628 m in the Eastern Pacific (12.7115′N, 103.9071′W). This strain M1-94P was determined to be S. xiamenensis by 16S rRNA gene sequence analysis.
4.3. Construction of Mutants by the Screening for Spontaneous Rifampicin Resistance
Fresh spores of wild strain M1-94P formed by cultivation on SFM (soy flour 20 g/L, d-mannitol 20 g/L, agar 1.5%, pH = 7.2) plates were incubated at 30 °C for 7 days and were then harvested and suspended in the appropriate amount of sterilized and distilled water. They were filtered to remove medium fragments and were then preserved in the 20% (v/v) glycerol tube (2 mL).
The M1-94P spore suspension was spread on GYM agar medium (glucose 4 g/L, yeast extract 4 g/L, malt extract 4 g/L, and agar 1.5% at pH = 7.2–7.4) containing various rifampicin concentrations. The MIC of rifampicin against M1-94P was determined through a two day incubation period at 30 °C on GYM agar medium. Spontaneous Rif mutants were obtained from the colonies that grew within 7–10 days after the spores of M1-94P were spread on the GYM agar medium containing different rifampicin concentrations (5 × MIC, 10 × MIC, 50 × MIC). Spontaneous drug resistant mutants were selected for further characterization.
4.4. Introduction of Streptomycin Resistance by Site Directed Mutagenesis
The rpsL gene fragment was amplified by PCR using the M1-94P genomic DNA as the template and was subjected to site-directed mutagenesis using PCR. The sequences of the forward primers were (mutagenic positions underlined): K88E-F (5′-GGCCGTGTGGAGGACCTGCCGGGTG-3′) and L90K-F (5′-GTGTGAAGGACAAGCCGGGTGTCCG-3′). The utilized reverse primers, K88E-R and L90K-R, were complementary to the forward primers. Primer pairs XJ1F (5′-CATATGGTGCCAACGATCCAGCA-3′) and XJ1R (5′-GATATCTTACTTCTCCTTCTTGGCGC-3′) were designed to introduce NdeI and EcoRV sites (underlined below) at the translation start and stop codon of the rpsL gene and combined with the primers K88E-F/R and L90K-F/R to generate point mutations in the mutagenesis PCR amplification.
The entire length of the mutated
rpsL gene was amplified by PCR and inserted into the pMD 18-T vector to check for site directed mutagenesis by sequencing, which generated plasmids p822 (K88E) and p827 (L90K). The
NdeI-
EcoRV fragments containing mutated
rpsL genes were excised and inserted into the same sites of pIB139, an integrative expression vector in
Streptomyces [
26], generating pIB139-822 (K88E) and pIB139-827 (L90K). These two plasmids were transformed into
E. coli ET12567: pUZ8002 separately and used as the donor strains for two parental
E. coli—
Streptomyces conjugations. Exoconjugants derived from the wild type
S. xiamenensis M1-5R22 were selected by rifampicin and apramycin on the SFM solid medium plates [
27]. The mutants harboring pIB139-822 and pIB139-827 were named M5 and M6, respectively.
4.5. Mutation Analysis of the rpsL and rpoB Genes
The primers for the PCR amplification of the rpoB and the rpsL genes were designed using the sequence information of the draft genome of S. xiamenensis (unpublished data). The partial rpoB gene fragments (nucleotides 374–1582, 1.2 kb) of the wild strain M1-94P and its Rif-resistant mutants (M1-5R22, M5 and M6) were obtained by PCR using their genomic DNA as templates and the synthetic oligonucleotide primers (forward: 5′-CCGAGTTCACCAACAACGAGACC-3′, reverse: 5′-CGATGACGAAGCGGTCCTCC-3′). The complete rpsL gene was amplified from the wild strain M1-94P and its drug-resistant mutants (M1-5R22, M5 and M6) by primer pairs (forward: 5′-TGTCCTCGGGTATCGGTCTG-3′ and reverse: 5′-TTACTTCTCCTTCTTGGCGCCGTAG-3′). The PCR products were directly sequenced by Sangon Biotech (Shanghai, China) Co., Ltd.
4.6. HPLC Analysis
Spore suspensions of the wild type strain M1-94P and its three mutants were inoculated in test tubes (15 × 150 mm) with 5 mL of Tryptone Soy Broth (TSB) (Oxoid, Hampshire, UK) and were pre-cultured at 30 °C for 1 day on a rotary shaker at 280 rpm. The TSB broth was then transferred into a 500 mL flask containing 100 mL of yeast extract-malt extract broth (GYM medium) and was cultured at 30 °C for 7 days on a rotary shaker at 280 rpm. Each culture was centrifuged at 9000 rpm for 10 min, and the supernatant was then extracted three times at room temperature overnight with equal volumes of the solvent ethyl acetate. The supernatant was combined and concentrated under vacuum at 37 °C to remove the organic phase. Each crude extract was subsequently dissolved in the same volume of HPLC grade methanol.
The samples were analyzed by HPLC using an Agilent XDB-C18 column (4.6 × 150 mm, 5 μm) and monitored by UV detection at 254 nm. The solvent system of methanol (A) and H2O (B) was used as the mobile phase in the following linear gradient: 0 min 10% B, 10 min 10% B, 40 min 100% B, 50 min 100% B, 51 min 10% B, 55 min 10% B, at a flow rate of 1 mL/min. The xiamenmycin obtained by our group was used as the standard reference.
4.7. Fermentation, Extraction, and Isolation
Mutant M6 was selected to perform a large-scale fermentation (33 L) in yeast extract-malt extract broth. The liquid culture was centrifuged at 9000 rpm for 10 min, and the supernatant was extracted at room temperature by ethyl acetate. The residue was extracted at room temperature overnight by a solvent mixture of ethyl acetate:methanol:acetic acid (80:5:5, v:v:v). The second supernatant was then filtered, and the residue was extracted twice more as described above. All the supernatants were combined and concentrated under vacuum at 37 °C to remove the organic phase. The crude extract (13.4 g) was obtained and was then subjected to silica gel column chromatography with elution by a mixture of dichloromethane (CH2Cl2) and methanol (MeOH) (gradient from CH2Cl2 (300 mL), 70:1 (v:v, 300 mL), 60:1 (v:v, 300 mL), 50:1 (v:v, 1.5 L), 30:1 (v:v, 800 mL), 10:1 (v:v, 1.5 L), 5:1 (v:v, 1 L), 2:1 (v:v, 1 L), 1:1 (v:v, 600 mL) to MeOH (600 mL)) and ten fractions (Fr.A–Fr.J) were obtained. Guided by HPLC fingerprinting, two fractions, Fr.F (3.09 g) eluted by CH2Cl2:MeOH = 15:1 to 10:1 and Fr.H (1.17 g) eluted by CH2Cl2:MeOH = 2:1, were collected and further subjected to Sephadex LH-20 column chromatography with elution by 100% methanol.
Fr.F-2 (0.478 g) was a subfraction of Fr.F, which was identified as the target fraction according to the HPLC fingerprints. Fr.F-2 was subsequently subjected to silica gel column chromatography and was eluted with CH2Cl2:MeOH. Fr.F-2-4 (eluted with CH2Cl2:MeOH (40:1, v:v)) showed the target peaks of the xiamenmycin derivatives from UV characterization at λmax = 206 and 260 nm. Fr.F-2-4 (18 mg) was further purified again by semi-preparative HPLC (Agilent ZOBRAX-C18 column, 5 μm, 9.4 × 250 mm), with the following gradient: CH3CN (A)/H2O (B): 0 min 42% A, 8 min 42% A, 33 min 72% A, 34 min 100% A, 50 min 100% A, at a flow rate of 1.5 mL/min. One sub-fraction Fr.F-2-4-2 contained compound 2 (1.5 mg). Fr.F-2-2 (8 mg) was subsequently purified by semi-preparative HPLC (Agilent ZOBRAX-C18 column, 5 μm, 9.4 × 250 mm), with the following gradient: CH3CN (A)/H2O (B): 0 min 45% A, 5 min 45% A, 35 min 55% A, 36 min 100% A, 50 min 100% A, at the flow rate of 1.5 mL/min. One sub fraction Fr.F-2-2-3 was collected as compound 1 (1.5 mg).
Compound
1: Yellow amorphous powder (MeOH); [α]
26D +28.45° (
c 0.0034, MeOH); UV (MeOH) λ
max = 206, 260 nm; CD (
c 0.0024, MeOH) Δε
201 +12.3, Δε
202 +9.6, Δε
205 +8.0, Δε
207.4 +7.1, Δε
213.6 +0.14, Δε
217 −1.2, Δε
245.2 +2.0, Δε
259.8 +3.14, Δε
283 +0.03;
1H and
13C NMR data, see
Table 2; HRESIMS
m/
z 290.1768 [M + H]
+, (calcd. for C
17H
24NO
3,
m/
z 290.1756), 288.1610 [M − H]
−, (calcd. for C
17H
22NO
3,
m/
z 288.2073).
Compound
2: Yellow amorphous powder (MeOH); [α]
30D +6.52° (
c 0.0024, MeOH); UV (MeOH) λ
max 206, 260 nm; CD (
c 0.0034, MeOH) Δε
205.6 +6.7, Δε
206.9 +5.5, Δε
213 +20.1, Δε
223.4 +0.85, Δε
260 +9.23, Δε
296.8 +1.0;
1H and
13C NMR data, see
Table 2; HRESIMS
m/
z 406.2210 [M + H]
+, (cacld. for C
22H
32NO
6,
m/
z 406.2230).
4.8. Cell Proliferation Assay
The effects of compounds 1 and 2, as well as xiamenmycin on cell proliferation were determined using a standard Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) assay according to the manufacturers instructions. WI26 cells at 70%–80% confluency were treated with a 0.25% trypsin and 0.02% EDTA solution, centrifuged and resuspended in DMEM supplemented with 10% FBS and antibiotics. The cells were then seeded in 96-well plates (100 μL/well) at an initial density of 2.5 × 104 cells/mL. The medium was replaced 24 h later by fresh DMEM with 10% FBS and antibiotics containing 15 μg/mL of compound 1, 30 μg/mL of compound 2, 30 μg/mL of xiamenmycin, or 0.1% DMSO (AppliChem, Darmstadt, Germany). Subsequently, the medium was refreshed and the viabilities of the cells were measured by using a CCK-8 solution at day 0, 1, 2, 3, 4, 5, and 6, respectively. A proliferation measurement was performed by adding 10 μL of CCK-8 solution to each well and incubating the solutions at 37 °C for 1 h. The OD values of each well were measured at the primary wavelength λ = 450 nm using a Microplate Spectrophotometer (PowerWaveXS, BioTek, Seattle, WA, USA). The data are shown as the means ± standard deviations (SD) of three independent experiments, each performed in triplicate.
4.9. Statistical Analysis
Statistical differences were calculated using student’s paired t-test at significance levels of p < 0.05 to 0.001.