Anti-Diabetic Effect of Balanced Deep-Sea Water and Its Mode of Action in High-Fat Diet Induced Diabetic Mice
Abstract
:1. Introduction
2. Results and Discussion
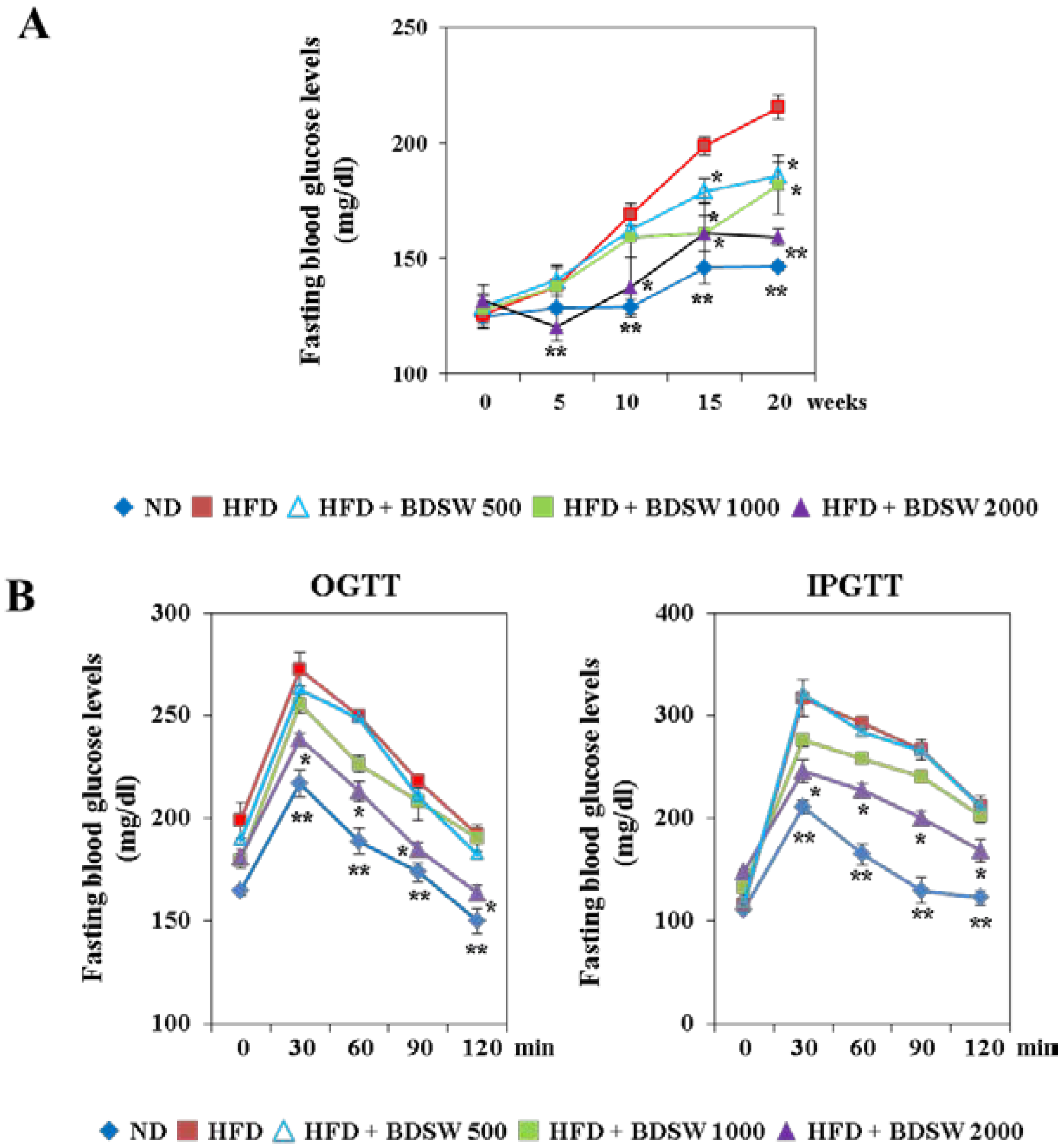
2.1. BDSW Suppresses Fasting Blood Glucose Levels and Improves Glucose Intolerance in High-Fat Diet (HFD)-Induced Diabetic Mice
| Measurement | ND | HFD | HFD + BDSW 500 | HFD + BDSW 1000 | HFD + BDSW 2000 |
|---|---|---|---|---|---|
| Initial body weight (g) | 18.9 ± 0.4 ** | 19.2 ± 0.3 | 18.7 ± 0.3 | 18.9 ± 0.1 | 19.0 ± 0.1 |
| Final body weight (g) | 25.3 ± 0.1 ** | 35.9 ± 0.6 | 33.7 ± 0.3 | 34.1 ± 0.4 | 33.2 ± 0.1 * |
| Weight gain (g/20 weeks/mouse) | 6.3 ± 1.0 ** | 16.74 ± 1.7 | 15.1 ± 2.5 | 15.2 ± 1.6 | 14.1 ± 1.4 * |
| Intra-abdominal fat weight (g/100 g body weight) | 1.8 ± 0.1 ** | 8.2 ± 0.2 | 8.1 ± 0.3 | 8.02 ± 0.1 | 7.6 ± 0.1 * |
| Food intake (g/day/mouse) | 2.5 ± 0.1 ** | 2.2 ± 0.1 | 2.3 ± 0.12 | 2.3 ± 0.1 | 2.2 ± 0.1 |
| Serum triglycerides | 45.6 ± 1.7 ** | 81.2 ± 6.4 | 65.9 ± 9.1 * | 61.5 ± 8.5 * | 49.6 ± 7.5 ** |
| Total cholesterol (mg/dL) | 78.9 ± 3.8 ** | 142.3 ± 4.3 | 131.52 ± 2.7 * | 133.8 ± 2.7 * | 126.7 ± 3.6 * |
| Liver triglycerides | 136.57 ± 1.6 ** | 246.5 ± 19.9 | 221.8 ± 23.6 | 195.8 ± 6.5 * | 187.5 ± 7.6 * |
| Total cholesterol (mg/g liver) | 99.8 ± 3.6 ** | 147.7 ± 5.1 | 149.2 ± 5.4 | 132.7 ± 7.1 * | 129.6 ± 5.1 * |
| Tap water & BDSW intake (mL/day/mouse) | 4.8 ± 0.1 ** | 3.9 ± 0.1 | 4.1 ± 0.1 | 4.2 ± 0.1 | 4.3 ± 0.1 |
| Serum insulin level (ng/mL) | 4.2 ± 0.8 | 3.6 ± 0.1 | 3.7 ± 0.3 | 5.4 ± 0.8 * | 6.3 ± 0.8 * |
2.2. Effect of BDSW on the Levels of Adiponectin, Leptin, and Insulin in the Plasma and Pancreas Functions of HFD-Induced Diabetic Mice

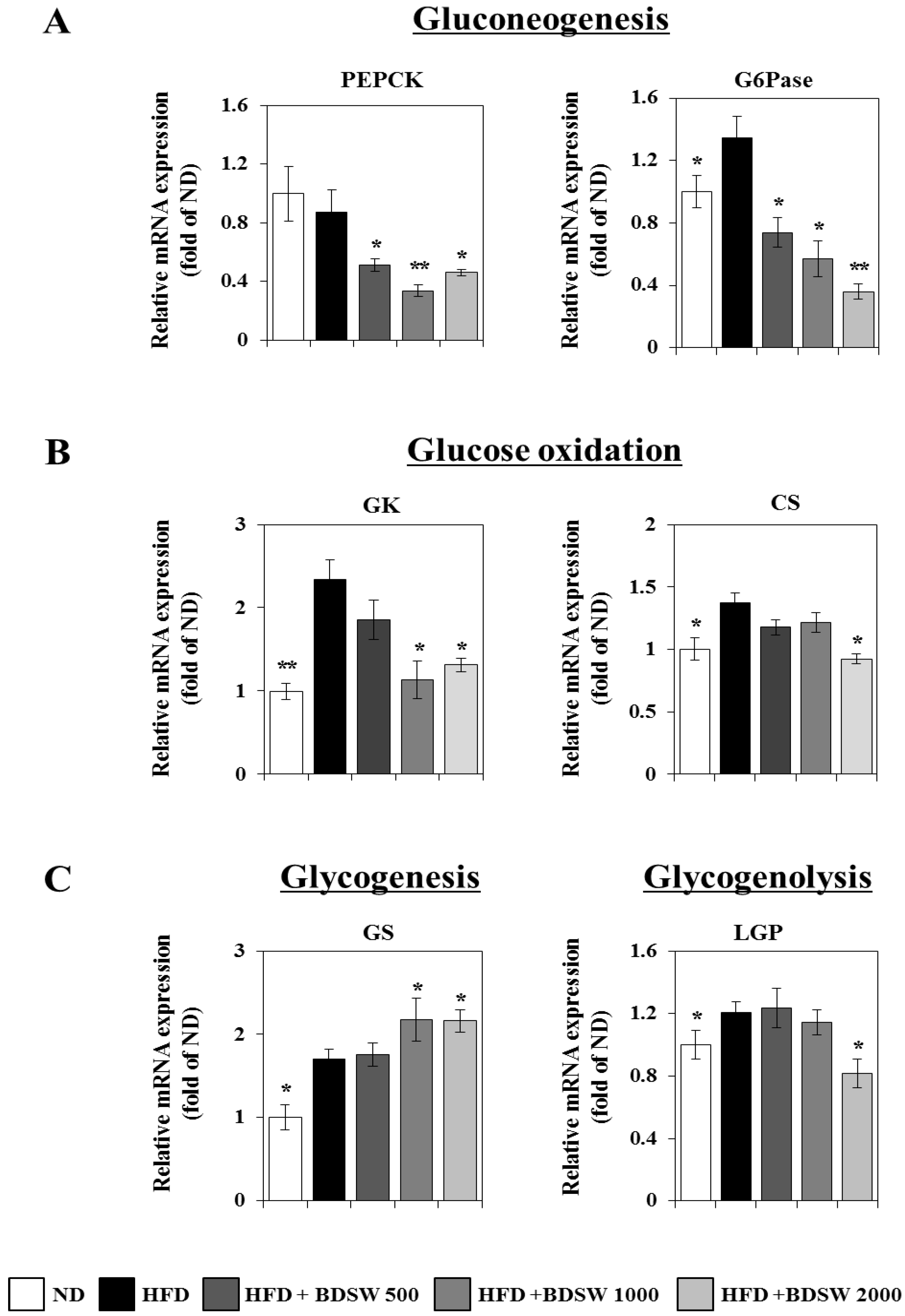
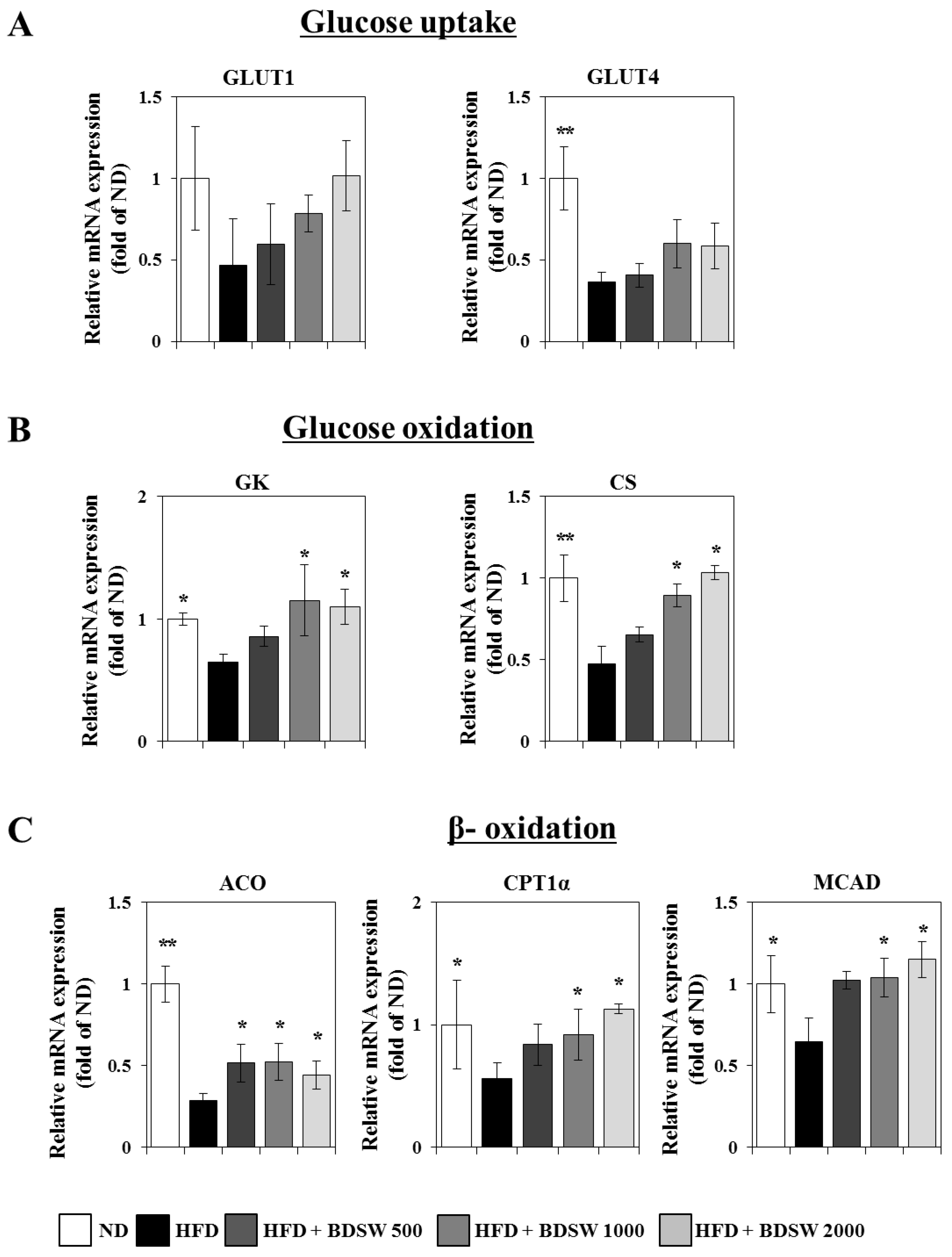
2.3. Effect of BDSW on the Expressions of Genes Related to Glucose Homeostasis in Livers and Muscles of HFD-Induced Diabetic Mice
2.4. BDSW Stimulates Glucose Uptake by 3T3-L1 Adipocytes in a Dose-Dependent Manner
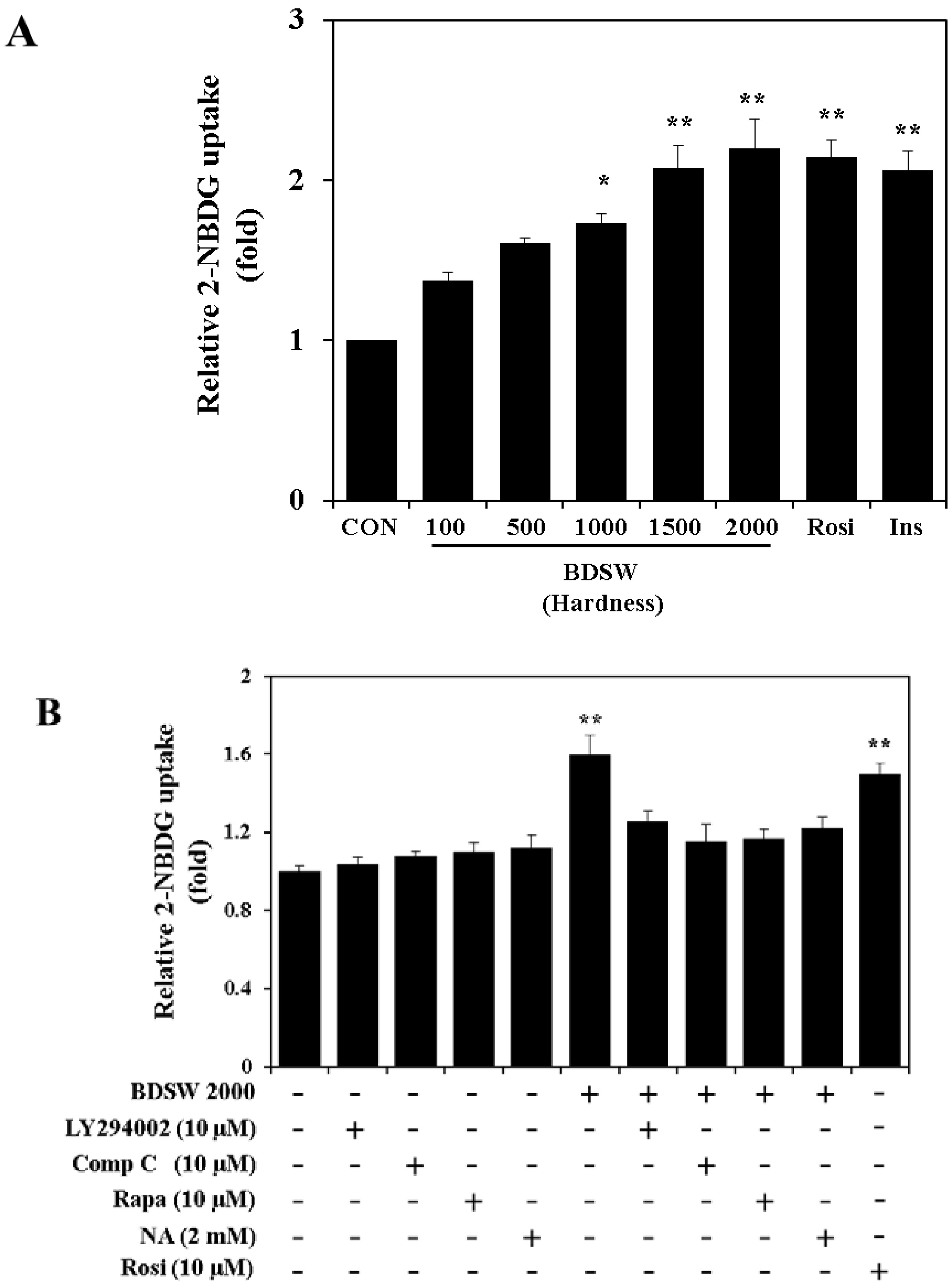
2.5. BDSW Activates the PI-3K and AMPK Signaling Pathways
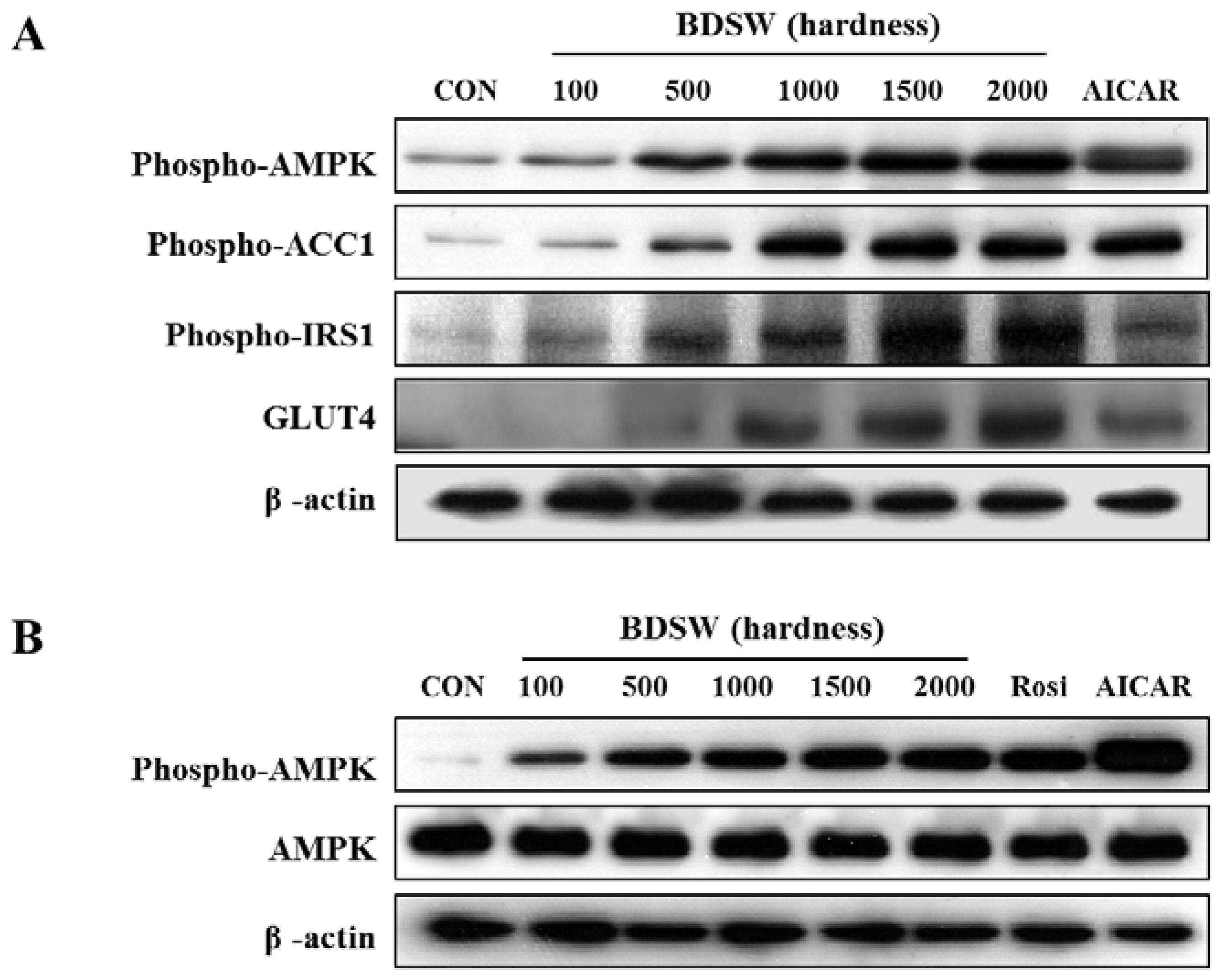
2.6. BDSW Specifically Stimulates AMPK Phosphorylation and Improves Impaired AMPK Phosphorylation in the Muscles and Livers of HFD-Induced Diabetic Mice
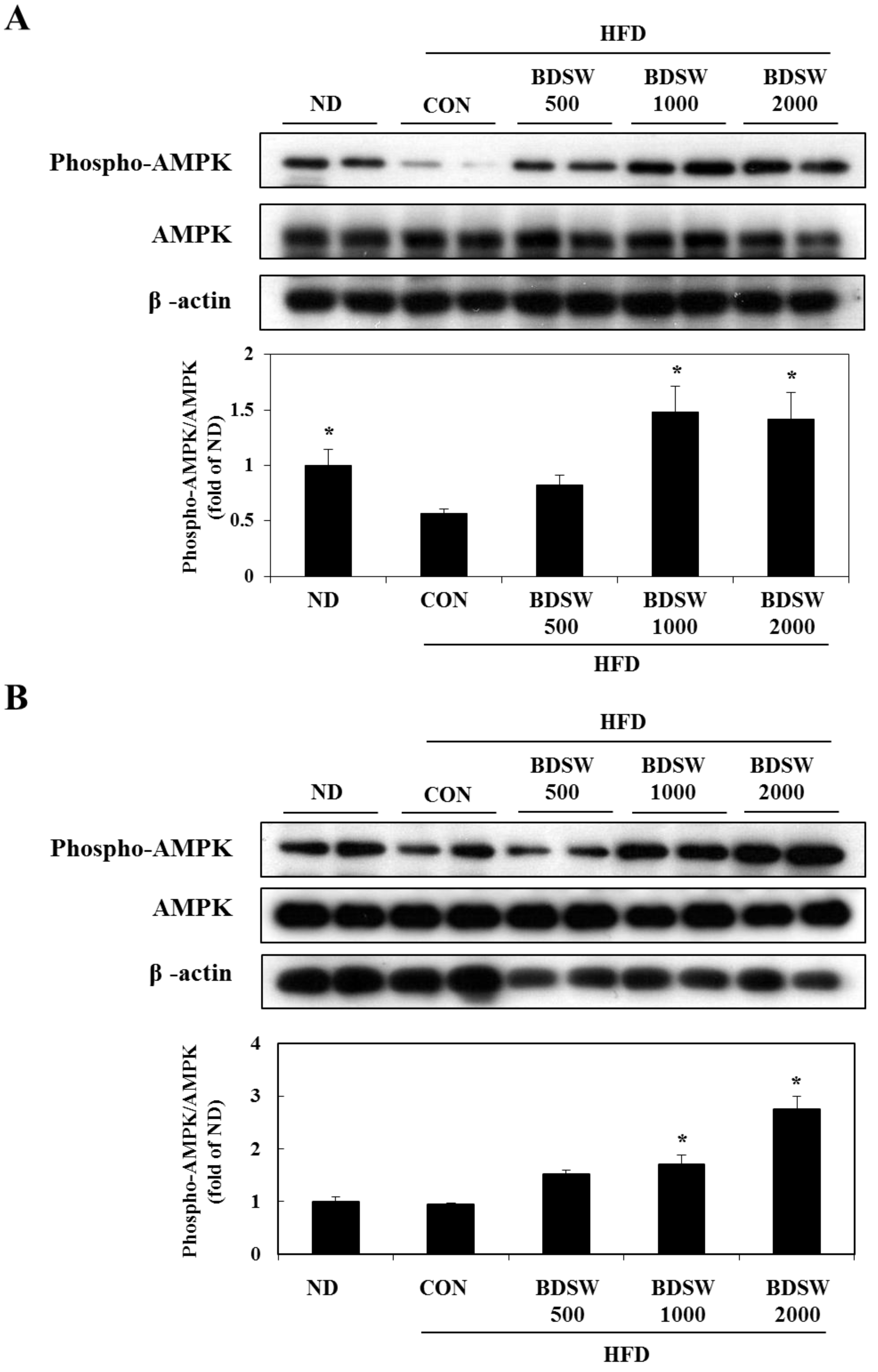
3. Experimental Section
3.1. Materials
3.2. The Preparatioin of BDSW
3.3. Animals and Diets
3.4. Determination of Blood Glucose Level
3.5. Cell Culture and Adipocyte Differentiation
3.6. Triglyceride and Cholesterol Assays of the Liver and Serum
3.7. Oral Glucose Tolerance Test (OGTT) and Intraperitoneal Glucose Tolerance Test (IPGTT)
3.8. Histopathological Analysis of Pancreas
3.9. Determination of Glucose Uptake by 3T3-L1 Adipocytes
3.10. Western Blot Analysis
3.11. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tahrani, A.A.; Piya, M.K.; Kennedy, A.; Barnett, A.H. Glycaemic control in type 2 diabetes: Targets and new therapies. Pharmacol. Ther. 2010, 125, 328–361. [Google Scholar]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar]
- Lee, T.I.; Chen, Y.C.; Kao, Y.H.; Hsiao, F.C.; Lin, Y.K.; Chen, Y.J. Rosiglitazone induces arrhythmogenesis in diabetic hypertensive rats with calcium handling alteration. Int. J. Cardiol. 2011, 165, 299–307. [Google Scholar]
- Moller, D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 2001, 414, 821–827. [Google Scholar]
- Liu, Q.; Chen, L.; Hu, L.; Guo, Y.; Shen, X. Small molecules from natural sources, targeting signaling pathways in diabetes. Biochim. Biophys. Acta 2010, 1799, 854–865. [Google Scholar]
- Yoshioka, S.; Hamada, A.; Cui, T.; Yokota, J.; Yamamoto, S.; Kusunose, M.; Miyamura, M.; Kyotani, S.; Kaneda, R.; Tsutsui, Y.; et al. Pharmacological activity of deep-sea water: Examination of hyperlipemia prevention and medical treatment effect. Biol. Pharm. Bull. 2003, 26, 1552–1559. [Google Scholar]
- Katsuda, S.; Yasukawa, T.; Nakagawa, K.; Miyake, M.; Yamasaki, M.; Katahira, K.; Mohri, M.; Shimizu, T.; Hazama, A. Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol. Pharm. Bull. 2008, 31, 38–44. [Google Scholar]
- Sheu, M.J.; Chou, P.Y.; Lin, W.H.; Pan, C.H.; Chien, Y.C.; Chung, Y.L.; Liu, F.C.; Wu, C.H. Deep sea water modulates blood pressure and exhibits hypolipidemic effects via the AMPK-ACC pathway: An in vivo study. Mar. Drugs 2013, 11, 2183–2202. [Google Scholar]
- Kimata, H.; Tai, H.; Nakagawa, K.; Yokoyama, Y.; Nakajima, H.; Ikegami, Y. Improvement of skin symptoms and mineral imbalance by drinking deep sea water in patients with atopic eczema/dermatitis syndrome (AEDS). Acta Medica 2002, 45, 83–84. [Google Scholar]
- Hataguchi, Y.; Tai, H.; Nakajima, H.; Kimata, H. Drinking deep-sea water restores mineral imbalance in atopic eczema/dermatitis syndrome. Eur. J. Clin. Nutr. 2005, 59, 1093–1096. [Google Scholar]
- Miyamura, M.; Yoshioka, S.; Hamada, A.; Takuma, D.; Yokota, J.; Kusunose, M.; Kyotani, S.; Kawakita, H.; Odani, K.; Tsutsui, Y.; et al. Difference between deep seawater and surface seawater in the preventive effect of atherosclerosis. Biol. Pharm. Bull. 2004, 27, 1784–1787. [Google Scholar]
- Resnick, L.M. Cellular calcium and manesium metabolism in the pathophysiology and treatment of hypertension and related metabolic disorders. Am. J. Med. 1992, 93, S11–S20. [Google Scholar]
- Robertson, D.S. Magnesium or calcium hypophosphite could be a treatment for obesity in humans. Med. Hypotheses 2006, 66, 439–440. [Google Scholar]
- Sales, C.H.; Pedrosa, L.F. Magnesium and diabetes mellitus: their relation. Clin. Nutr. 2006, 25, 554–562. [Google Scholar]
- Hwang, H.S.; Kim, H.A.; Lee, S.H.; Yun, J.W. Anti-obesity and antidiabetic effects of deep sea water on ob/ob mice. Mar. Biotechnol. 2009, 11, 531–539. [Google Scholar]
- Hwang, H.S.; Kim, S.H.; Yoo, Y.G.; Chu, Y.S.; Shon, Y.H.; Nam, K.S.; Yun, J.W. Inhibitory effect of deep-sea water on differentiation of 3T3-L1 adipocytes. Mar. Biotechnol. 2009, 11, 161–168. [Google Scholar]
- Radhakrishnan, G.; Yamamoto, M.; Maeda, H.; Nakagawa, A.; Kataregopalrao, R.; Okada, H.; Nishimori, H.; Wariishi, S.; Toda, E.; Ogawa, H.; et al. Intake of dissolved organic matter from deep seawater inhibits atherosclerosis progression. Biochem. Biophys. Res. Commun. 2009, 387, 25–30. [Google Scholar]
- Fu, Z.Y.; Yang, F.L.; Hsu, H.W.; Lu, Y.F. Drinking deep seawater decreases serum total and low-density lipoprotein-cholesterol in hypercholesterolemic subjects. J. Med. Food 2012, 15, 535–541. [Google Scholar]
- Kushner, R.F.; Sujak, M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity 2009, 17, 1017–1022. [Google Scholar]
- Lottenberg, A.M.; Afonso, M.S.; Lavrador, M.S.; Machado, R.M.; Nakandakare, E.R. The role of dietary fatty acids in the pathology of metabolic syndrome. J. Nutr. Biochem. 2012, 23, 1027–1040. [Google Scholar]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar]
- Okamoto, Y.; Kihara, S.; Funahashi, T.; Matsuzawa, Y.; Libby, P. Adiponectin: A key adipocytokine in metabolic syndrome. Clin. Sci. 2006, 110, 267–278. [Google Scholar]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar]
- Tschop, M.; Heiman, M.L. Rodent obesity models: an overview. Exp. Clin. Endocrinol. Diabetes 2001, 109, 307–319. [Google Scholar]
- Lin, S.; Thomas, T.C.; Storlien, L.H.; Huang, X.F. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 639–646. [Google Scholar]
- Raucci, R.; Rusolo, F.; Sharma, A.; Colonna, G.; Castello, G.; Costantini, S. Functional and structural features of adipokine family. Cytokine 2013, 61, 1–14. [Google Scholar]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Omar, B.; Pacini, G.; Ahren, B. Differential development of glucose intolerance and pancreatic islet adaptation in multiple diet induced obesity models. Nutrients 2012, 4, 1367–1381. [Google Scholar]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar]
- Schug, T.T.; Li, X. Sirtuin 1 in lipid metabolism and obesity. Ann. Med. 2011, 43, 198–211. [Google Scholar]
- Cok, A.; Plaisier, C.; Salie, M.J.; Oram, D.S.; Chenge, J.; Louters, L.L. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 2011, 93, 1187–1192. [Google Scholar]
- Zhang, W.Y.; Lee, J.J.; Kim, I.S.; Kim, Y.; Park, J.S.; Myung, C.S. 7-O-methylaromadendrin stimulates glucose uptake and improves insulin resistance in vitro. Biol. Pharm. Bull. 2010, 33, 1494–1499. [Google Scholar]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar]
- Hurley, R.L.; Anderson, K.A.; Franzone, J.M.; Kemp, B.E.; Means, A.R.; Witters, L.A. The Ca2+/Calmodulin-dependent Protein Kinase Kinases Are AMP-activated Protein Kinase Kinases. J. Biol. Chem. 2005, 280, 29060–29066. [Google Scholar]
- Anderson, K.A.; Means, R.L.; Huang, Q.H.; Kemp, B.E.; Goldstein, E.G.; Selbert, M.A.; Edelman, A.M.; Fremeau, R.T.; Means, A.R. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biol. Chem. 1998, 273, 31880–31889. [Google Scholar]
- Anderson, K.A.; Ribar, T.J.; Lin, F.; Noeldner, P.K.; Green, M.F.; Muehlbauer, M.J.; Witters, L.A.; Kemp, B.E.; Means, A.R. Hypothalamic CaMKK2 Contributes to the Regulation of Energy Balance. Cell Metab. 2008, 7, 377–388. [Google Scholar]
- Witters, L.A.; Kemp, B.E.; Means, A.R. Chutes and Ladders: The search for protein kinases that act on AMPK. Trends Biochem. Sci. 2006, 31, 13–16. [Google Scholar]
- Jung, D.W.; Ha, H.H.; Zheng, X.; Chang, Y.T.; Williams, D.R. Novel use of fluorescent glucose analogues to identify a new class of triazine-based insulin mimetics possessing useful secondary effects. Mol. Biosyst. 2011, 2, 346–358. [Google Scholar]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ha, B.G.; Shin, E.J.; Park, J.-E.; Shon, Y.H. Anti-Diabetic Effect of Balanced Deep-Sea Water and Its Mode of Action in High-Fat Diet Induced Diabetic Mice. Mar. Drugs 2013, 11, 4193-4212. https://doi.org/10.3390/md11114193
Ha BG, Shin EJ, Park J-E, Shon YH. Anti-Diabetic Effect of Balanced Deep-Sea Water and Its Mode of Action in High-Fat Diet Induced Diabetic Mice. Marine Drugs. 2013; 11(11):4193-4212. https://doi.org/10.3390/md11114193
Chicago/Turabian StyleHa, Byung Geun, Eun Ji Shin, Jung-Eun Park, and Yun Hee Shon. 2013. "Anti-Diabetic Effect of Balanced Deep-Sea Water and Its Mode of Action in High-Fat Diet Induced Diabetic Mice" Marine Drugs 11, no. 11: 4193-4212. https://doi.org/10.3390/md11114193




