LC-PUFA-Enriched Oil Production by Microalgae: Accumulation of Lipid and Triacylglycerols Containing n-3 LC-PUFA Is Triggered by Nitrogen Limitation and Inorganic Carbon Availability in the Marine Haptophyte Pavlova lutheri
Abstract
:1. Introduction
2. Results and Discussion
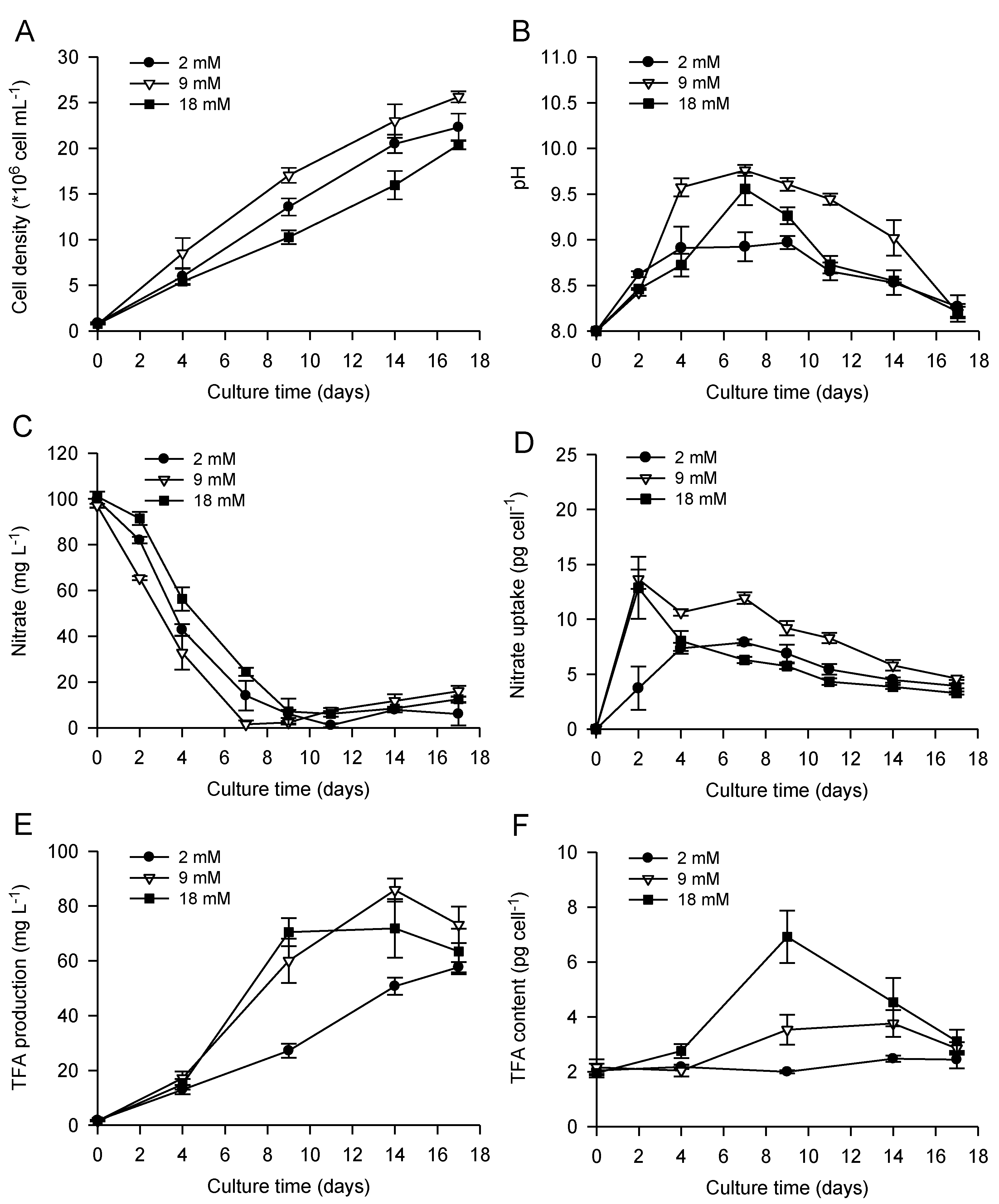
2.1. Bicarbonate Addition Promotes Growth, Nitrate Uptake and Lipid Production during Batch Cultivation of P. lutheri
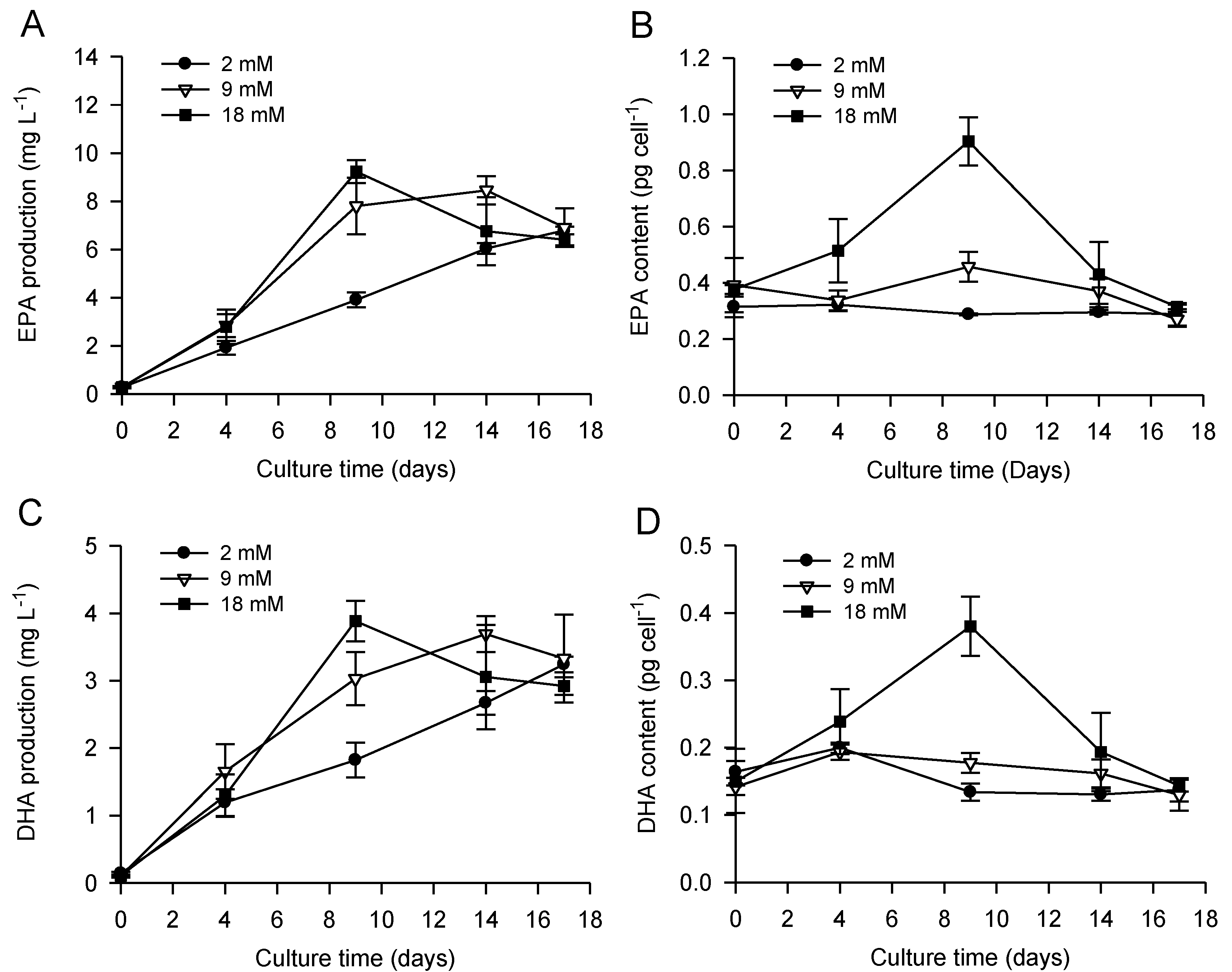
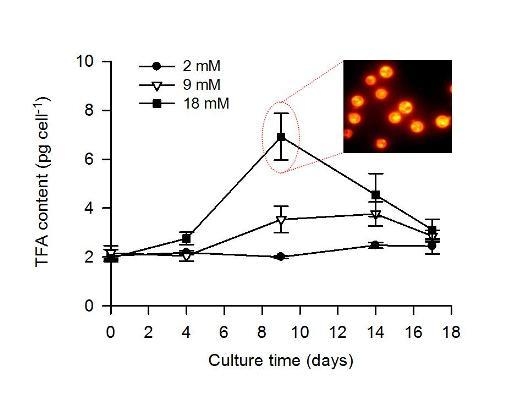
2.2. Combined Effect of Nitrogen Limitation and Bicarbonate Addition on Total Fatty Acid Composition and n-3 LC-PUFA Production
| Bicarbonate (mM) | ||||||
|---|---|---|---|---|---|---|
| Before N-Limitation | After N-Limitation | |||||
| Fatty Acids (% TFA) | 2 | 9 | 18 | 2 | 9 | 18 |
| Saturated fatty acids | ||||||
| 14:0 | 17.3 ± 0.7 | 15.2 ± 0.7 | 15.5 ± 2.8 | 14.3 ± 0.3 | 13.8 ± 0.3 | 14.7 ± 1.3 |
| 16:0 | 18.5 ± 1.1 | 19.7 ± 0.3 | 19.0 ± 2.0 | 26.2 ± 0.1 | 27.4 ± 0.8 | 29.9 ± 1.9 |
| 18:0 | 2.1 ± 1.8 | 1.9 ± 0.2 | 2.7 ± 2.5 | 0.6 ± 0.0 | 1.5 ± 0.3 | 0.7 ± 0.2 |
| Sum of SFA | 37.8 ± 0.4 | 36.9 ± 1.0 | 37.2 ± 7.2 | 41.1 ± 0.2 | 42.6 ± 0.2 | 45.3 ± 0.6 |
| Monounsaturated fatty acids | ||||||
| 16:1 n-7 | 24.6 ± 1.0 | 20.2 ± 1.3 | 17.9 ± 0.5 | 30.3 ± 0.1 | 29.4 ± 0.6 | 29.3 ± 1.6 |
| 18:1 n-7 | 1.3 ± 0.2 | 1.0 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.1 | 2.2 ± 0.2 | 1.4 ± 0.1 |
| 18:1 n-9 | 2.0 ± 0.1 | 2.6 ± 0.6 | 2.0 ± 0.1 | 2.1 ± 0.1 | 2.9 ± 0.2 | 1.5 ± 0.2 |
| Sum of MUFA | 27.8 ± 0.9 | 23.9 ± 1.4 | 21.2 ± 0.3 | 33.9 ± 0.3 | 34.4 ± 0.8 | 32.2 ± 1.4 |
| Polyunsaturated fatty acids | ||||||
| 18:2 n-6 | 0.8 ± 0.1 | 1.2 ± 0.2 | 1.4 ± 0.3 | 1.6 ± 0.1 | 2.0 ± 0.1 | 1.9 ± 0.2 |
| 18:3 n-6 | tr. | 0.6 ± 0.1 | tr. | tr. | 0.3 ± 0.0 | tr. |
| 18:3 n-3 | 1.1 ± 0.3 | 1.1 ± 0.2 | 0.4 ± 0.1 | 0.7 ± 0.0 | 0.8 ± 0.1 | 0.6 ± 0.0 |
| 18:4 n-3 | 4.0 ± 0.5 | 5.7 ± 0.1 | 7.2 ± 1.4 | 2.0 ± 0.0 | 2.0 ± 0.2 | 2.6 ± 0.1 |
| 20:4 n-6 | 0.8 ± 0.1 | 0.6 ± 0.3 | 0.9 ± 0.2 | 0.9 ± 0.3 | 1.0 ± 0.1 | 0.8 ± 0.2 |
| 20:5 n-3 | 16.1 ± 0.2 | 16.5 ± 0.4 | 18.7 ± 4.1 | 11.6 ± 0.2 | 9.8 ± 0.6 | 9.4 ± 1.0 |
| 22:5 n-3 | 0.8 ± 0.3 | 0.9 ± 0.2 | 0.6 ± 0.0 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.5 ± 0.3 |
| 22:6 n-3 | 9.0 ± 0.3 | 9.6 ± 1.4 | 8.6 ± 1.5 | 5.3 ± 0.1 | 4.3 ± 0.3 | 4.2 ± 0.4 |
| Sum of PUFA | 32.3 ± 0.5 | 36.1 ± 1.4 | 37.9 ± 6.9 | 23.4 ± 0.5 | 21.3 ± 1.1 | 21.0 ± 1.6 |
| Others | 2.0 ± 0.4 | 3.2 ± 0.3 | 3.7 ± 0.6 | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 |
| n-3 | 29.9 ± 0.8 | 33.8 ± 1.5 | 35.5 ± 7.1 | 20.1 ± 0.3 | 18.1 ± 1.1 | 18.3 ± 1.2 |
| n-6 | 2.4 ± 0.4 | 2.3 ± 0.2 | 2.3 ± 0.1 | 3.3 ± 0.3 | 3.3 ± 0.1 | 2.7 ± 0.3 |
| TFA (pg cell−1) | 2.2 ± 0.1 | 2.0 ± 0.2 | 2.8 ± 0.3 | 2.5 ± 0.1 | 3.8 ± 0.5 | 6.9 ± 1.0 |
| TFA (mg L−1) | 13.0 ± 1.7 | 17.2 ± 2.5 | 14.9 ± 1.9 | 57.7 ± 1.9 | 85.8 ± 4.2 | 71.9 ± 10.7 |
2.3. Increased TAG Accumulation and Oil-Body Formation Is to a Large Extent Attributable to Increased Carbon Availability
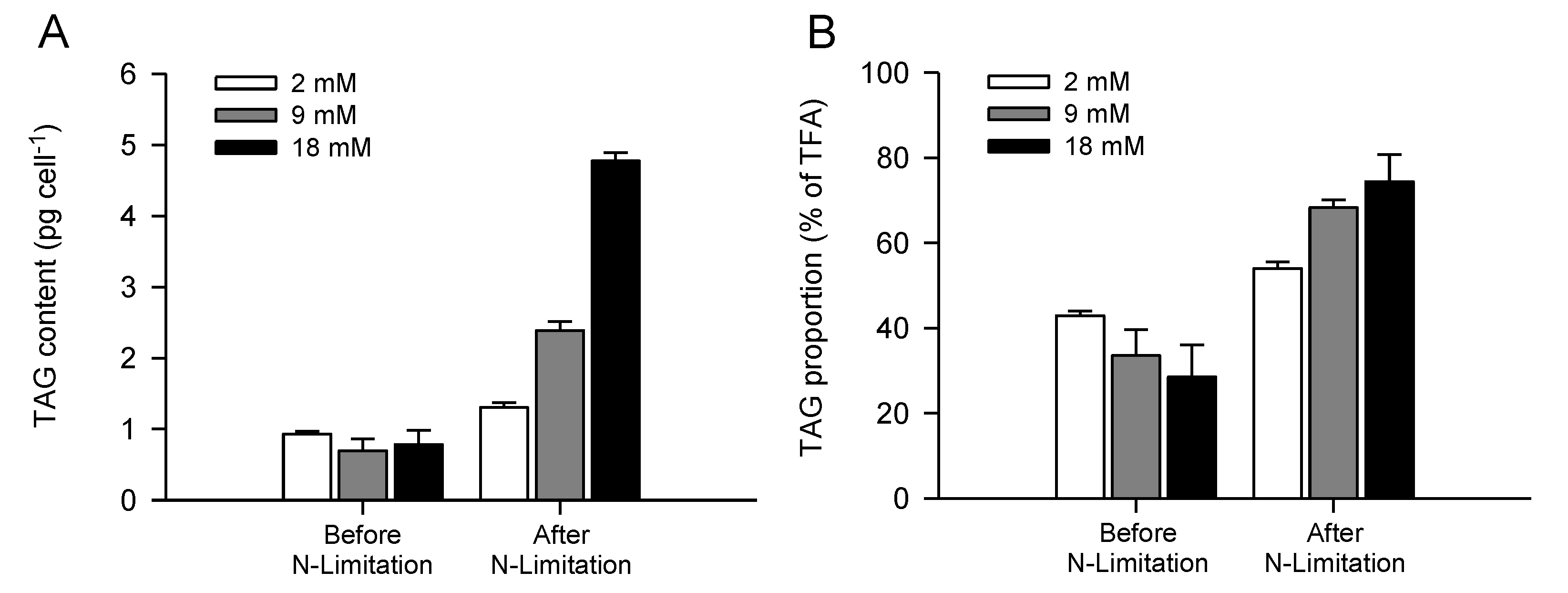

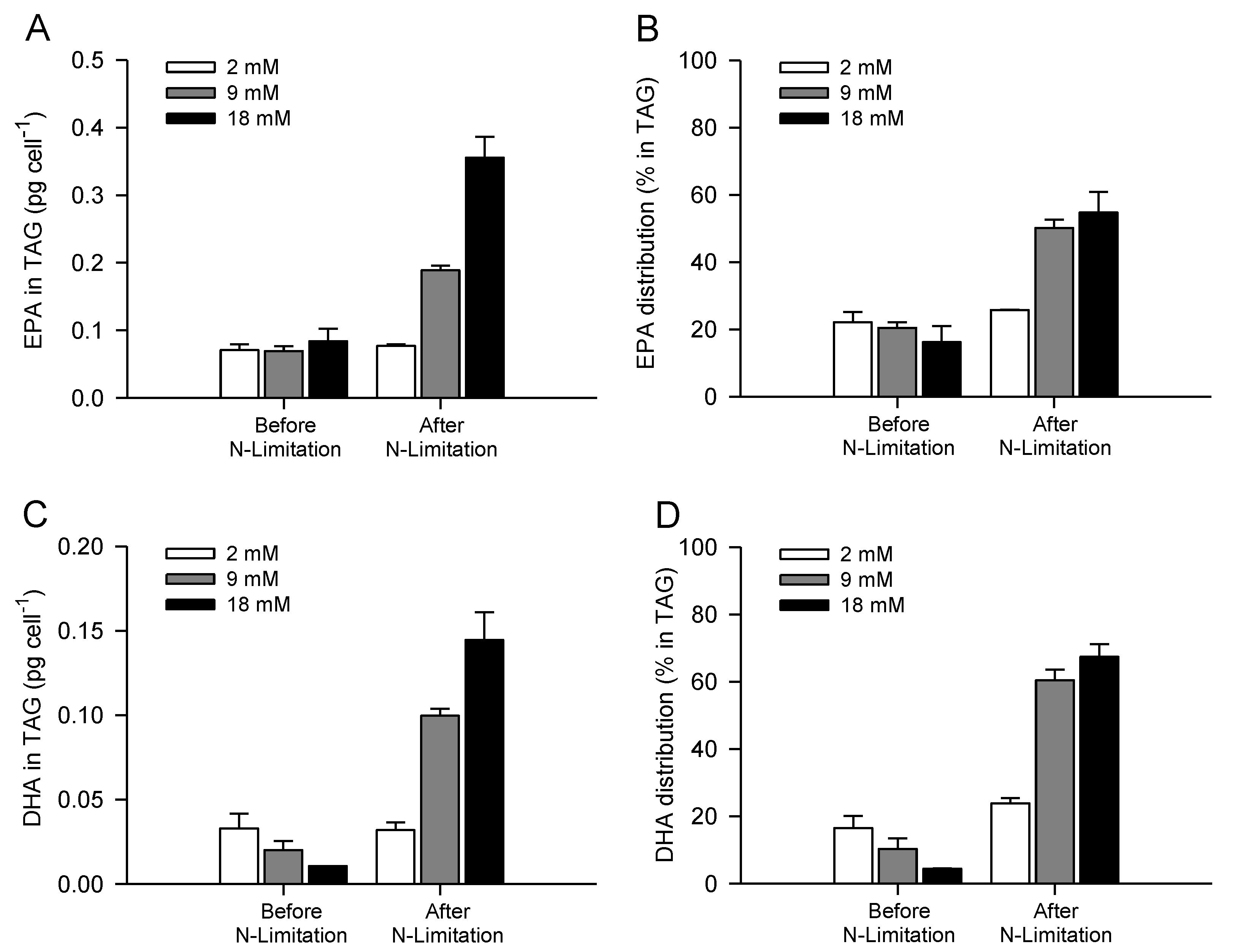
2.4. Increasing Inorganic Carbon Supply Enhances Accumulation of TAG Containing n-3 LC-PUFA
| Bicarbonate (mM) | ||||||
|---|---|---|---|---|---|---|
| Before N-Limitation | After N-Limitation | |||||
| Fatty Acids (% TFA) | 2 | 9 | 18 | 2 | 9 | 18 |
| Saturated fatty acids | ||||||
| 14:0 | 11.2 ± 0.8 | 9.6 ± 0.9 | 9.6 ± 0.9 | 10.7 ± 0.6 | 9.7 ± 0.2 | 10.3 ± 0.4 |
| 16:0 | 22.6 ± 2.1 | 22.4 ± 1.3 | 22.4 ± 1.3 | 30.7 ± 0.8 | 27.4 ± 0.3 | 29.5 ± 0.5 |
| 18:0 | 5.6 ± 1.4 | 5.5 ± 1.7 | 5.5 ± 1.7 | 0.8 ± 0.1 | 0.9 ± 1.2 | 0.9 ± 0.2 |
| Sum of SFA | 39.5 ± 0.1 | 37.5 ± 3.9 | 37.5 ± 3.9 | 42.2 ± 1.4 | 38.1 ± 0.3 | 40.7 ± 1.4 |
| Monounsaturated fatty acids | ||||||
| 16:1 n-7 | 22.0 ± 3.3 | 22.9 ± 10.3 | 15.9 ± 0.3 | 36.5 ± 0.2 | 33.9 ± 0.1 | 30.8 ± 0.5 |
| 18:1 n-7 | 3.0 ± 0.9 | 4.5 ± 2.2 | 3.7 ± 0.5 | 2.0 ± 0.0 | 1.7 ± 0.1 | 1.8 ± 0.3 |
| 18:1 n-9 | 5.6 ± 2.1 | 4.5 ± 1.1 | 5.4 ± 0.8 | 2.1 ± 0.1 | 3.3 ± 0.2 | 2.4 ± 0.0 |
| Sum of MUFA | 30.7 ± 2.1 | 31.8 ± 7.1 | 24.9 ± 0.6 | 40.7 ± 0.2 | 38.9 ± 0.2 | 35.0 ± 0.2 |
| Polyunsaturated fatty acids | ||||||
| 18:2 n-6 | 3.5 ± 0.3 | 3.4 ± 0.3 | 3.9 ± 0.3 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.0 |
| 18:3 n-6 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.8 ± 0.4 | 0.4 ± 0.0 | 0.6 ± 0.1 | 0.4 ± 0.1 |
| 18:3 n-3 | 1.2 ± 0.2 | 1.7 ± 0.2 | 2.0 ± 1.2 | 2.0 ± 0.0 | 2.4 ± 0.1 | 3.0 ± 0.2 |
| 18:4 n-3 | 1.5 ± 0.4 | 2.1 ± 0.3 | 1.7 ± 0.0 | 1.0 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 |
| 20:4 n-6 | 1.3 ± 0.3 | 0.9 ± 0.2 | 1.6 ± 0.3 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 |
| 20:5 n-3 | 7.6 ± 0.6 | 10.1 ± 1.4 | 10.8 ± 0.4 | 5.9 ± 0.5 | 7.9 ± 0.1 | 8.2 ± 0.5 |
| 22:5 n-3 | 0.4 ± 0.0 | 0.6 ± 0.0 | 0.7 ± 0.1 | 0.7 ± 0.1 | 1.1 ± 0.2 | 1.5 ± 0.1 |
| 22:6 n-3 | 3.5 ± 0.8 | 2.9 ± 0.1 | 1.4 ± 0.4 | 2.5 ± 0.5 | 4.2 ± 0.4 | 4.2 ± 0.3 |
| Sum of PUFA | 19.3 ± 1.4 | 22.1 ± 2.2 | 23.0 ± 1.6 | 13.9 ± 1.3 | 19.2 ± 0.6 | 20.6 ± 1.2 |
| Others | 10.6 ± 3.5 | 8.6 ± 1.0 | 16.9 ± 3.8 | 3.3 ± 0.3 | 3.8 ± 0.1 | 3.8 ± 0.1 |
| n-3 | 14.3 ± 1.9 | 17.5 ± 1.8 | 16.7 ± 1.8 | 10.4 ± 1.2 | 15.3 ± 0.8 | 15.9 ± 0.9 |
| n-6 | 5.0 ± 0.6 | 4.6 ± 0.4 | 6.3 ± 0.2 | 3.4 ± 0.1 | 3.9 ± 0.2 | 4.6 ± 0.3 |
| TAG (pg cell−1) | 0.9 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.2 | 1.3 ± 0.1 | 2.4 ± 0.1 | 4.8 ± 0.1 |
| TAG (% TFA) | 42.9 ± 1.1 | 33.6 ± 6.1 | 28.5 ± 7.6 | 54.0 ± 1.6 | 68.3 ± 1.9 | 74.4 ± 6.4 |
3. Experimental Section
3.1. Strain and Culture Conditions
3.2. Growth Parameters, Medium pH, Biomass Harvesting and Storage
3.3. Nitrate Determination
3.4. Nile Red Staining and Microscopy
3.5. Lipid Extraction and Analysis
3.6. Fatty Acid Analysis
4. Conclusions
Abbreviations
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| LC-PUFA | long-chain polyunsaturated fatty acids |
| PUFA | polyunsaturated fatty acids |
| TAG | triacylglycerols |
Acknowledgments
Conflicts of Interest
References
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sust. Energ. Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2013, 13, 2733–2750. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Worm, B.; Hilborn, R.; Baum, J.K.; Branch, T.A.; Collie, J.S.; Costello, C.; Fogarty, M.J.; Fulton, E.A.; Hutchings, J.A.; Jennings, S.; et al. Rebuilding global fisheries. Science 2009, 325, 578–585. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Iskandarov, U.; Cohen, Z. LC-PUFA from photosynthetic microalgae: occurrence, biosynthesis, and prospects in biotechnology. Appl. Microbiol. Biotechnol. 2011, 91, 905–915. [Google Scholar] [CrossRef]
- Martins, D.A.; Custódio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K.M. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar. Drugs 2013, 11, 2259–2281. [Google Scholar] [CrossRef]
- Berge, J.P.; Gouygou, J.P.; Dubacq, J.P.; Durand, P. Reassessment of lipid composition of the diatom, Skeletonema costatum. Phytochemistry 1995, 39, 1017–1021. [Google Scholar] [CrossRef]
- Alonso, D.L.; Belarbi, E.H.; Rodriguez-Ruiz, J.; Segura, C.I.; Gimenez, A. Acyl lipids of three microalgae. Phytochemistry 1998, 47, 1473–1483. [Google Scholar] [CrossRef]
- Tonon, T.; Harvey, D.; Larson, T.R.; Graham, I.A. Long chain polyunsaturated fatty acid production and partitioning to triacylglycerols in four microalgae. Phytochemistry 2002, 61, 15–24. [Google Scholar]
- Guihéneuf, F.; Fouqueray, M.; Mimouni, V.; Ulmann, L.; Jacquette, B.; Tremblin, G. Effect of UV stress on the fatty acid and lipid class composition in two marine microalgae Pavlova lutheri (Pavlovophyceae) and Odontella aurita (Bacillariophyceae). J. Appl. Phycol. 2010, 22, 629–638. [Google Scholar] [CrossRef]
- Meireles, L.A.; Guedes, A.C.; Malcata, F.X. Lipid class composition of the microalga Pavlova lutheri: eicosapentaenoic and docosahexaenoic acids. J. Agric. Food Chem. 2003, 51, 2237–2241. [Google Scholar] [CrossRef]
- Bigogno, C.; Khozin-Goldberg, I.; Boussiba, S.; Vonshak, A.; Cohen, Z. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 2002, 5, 497–503. [Google Scholar]
- Khozin-Goldberg, I.; Bigogno, C.; Shrestha, P.; Cohen, Z. Nitrogen starvation induces the accumulation of arachidonic acid in the freshwater green alga Parietochloris incisa (Trebouxiophyceae). J. Phycol. 2002, 38, 991–994. [Google Scholar] [CrossRef]
- Yu, E.T.; Zendejas, F.J.; Lane, P.D.; Gaucher, S.; Simmons, B.A.; Lane, T.W. Triacylglycerol accumulation and profiling in the model diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum (Baccilariophyceae) during starvation. J. Appl. Phycol. 2009, 21, 669–681. [Google Scholar] [CrossRef]
- Roessler, P.G. Effects of silicon deficiency on lipid composition and metabolism in the diatom Cyclotella cryptica. J. Phycol. 1988, 24, 394–400. [Google Scholar]
- Khozing-Goldberg, I.; Cohen, Z. The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 2006, 67, 696–701. [Google Scholar]
- Gong, Y.; Guo, X.; Wan, X.; Liang, Z.; Jiang, M. Triacylglycerol accumulation and change in fatty acid content of four marine oleaginous microalgae under nutrient limitation and at different culture ages. J. Basic Microb. 2013, 53, 29–36. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar]
- Breuer, G.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour. Technol. 2012, 124, 217–226. [Google Scholar] [CrossRef]
- Mortensen, S.H.; Borsheim, K.Y.; Rainuzzo, J.; Knutsen, G. Fatty acid and elemental composition of the marine diatom Chaetoceros gracilis Schutt. Effects of silicate deprivation, temperature and light intensity. J. Exp. Mar. Biol. Ecol. 1988, 122, 173–185. [Google Scholar] [CrossRef]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar]
- Fidalgo, J.P.; Cid, A.; Torres, E.; Sukenik, A.; Herrero, C. Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture 1998, 166, 105–116. [Google Scholar] [CrossRef]
- Roncarati, A.; Meluzzi, A.; Acciarri, S.; Tallarico, N.; Meloti, P. Fatty acid composition of different microalgae strains (Nannochloropsis sp., Nannochloropsis oculata (Droop) Hibberd, Nannochloris atomus Butcher and Isochrysis sp.) according to the culture phase and the carbon dioxide concentration. J. World Aquacult. Soc. 2004, 35, 401–411. [Google Scholar] [CrossRef]
- Illman, A.L.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Tech. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Wu, W.-T. Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour. Technol. 2009, 100, 3921–3926. [Google Scholar] [CrossRef]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J. Appl. Phycol. 2012, 24, 989–1001. [Google Scholar] [CrossRef]
- Kaplan, D.; Richmond, A.E.; Dubinsky, Z.; Aaronson, S. Algal nutrition. In Handbook of Microalgal Mass Culture; Richmond, A., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 147–198. [Google Scholar]
- Tsuzuki, M.; Ohnuma, E.; Sato, N.; Takaku, T.; Kawaguchi, A. Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiol. 1990, 93, 851–856. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Goutx, M.; Figueroa, F.L.; Niell, F.X. Effects of light intensity, CO2 and nitrogen supply on lipid class composition of Dunaliella viridis. J. Appl. Phycol. 1998, 10, 135–144. [Google Scholar] [CrossRef]
- Hu, H.; Gao, K. Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol. Lett. 2006, 28, 987–992. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Malcata, F.X. Optimization of ω-3 fatty acid production by microalgae: Crossover effects of CO2 and light intensity under batch and continuous cultivation modes. Mar. Biotechnol. 2005, 7, 381–388. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-H. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef]
- Tang, H.; Abunasser, N.; Garcia, M.E.D.; Chen, M.; Simon Ng, K.Y.; Salley, S.O. Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Appl. Energ. 2011, 88, 3324–330. [Google Scholar] [CrossRef]
- Hsueh, H.T.; Chu, H.; Yu, S.T. A batch study on the bio-fixation of carbon dioxide in the absorbed solution from a chemical wet scrubber by hot spring and marine algae. Chemosphere 2007, 66, 878–886. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Mimouni, V.; Ulmann, L.; Tremblin, G. Combined effects of irradiance level and carbon source on fatty acid and lipid class composition in the microalga Pavlova lutheri commonly used in mariculture. J. Exp. Mar. Biol. Ecol. 2009, 369, 136–143. [Google Scholar] [CrossRef]
- Gardner, R.D.; Cooksey, K.E.; Mus, F.; Macur, R.; Moll, K.; Eustance, E.; Carlson, R.P.; Gerlach, R.; Fields, M.W.; Peyton, B.M. Use of sodium bicarbonate to stimulate triacylglycerol accumulation in the chlorophyte Scenedesmus sp. and the diatom Phaeodactylum tricornutum. J. Appl. Phycol. 2012, 24, 1311–1320. [Google Scholar]
- Gardner, R.D.; Lohman, E.; Gerlach, R.; Cooksey, K.E.; Peyton, B.M. Comparison of CO2 and bicarbonate as inorganic carbon sources for triacylglycerol and starch accumulation in Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2013, 110, 87–96. [Google Scholar] [CrossRef]
- White, D.A.; Pagarette, A.; Rooks, P.; Ali, S.T. The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J. Appl. Phycol. 2013, 25, 153–165. [Google Scholar]
- Mus, F.; Toussaint, J.-P.; Cooksey, K.E.; Fields, M.W.; Gerlach, R.; Peyton, B.M.; Carlson, R.P. Physiological and molecular analysis of carbon source supplementation and pH stress-induced lipid accumulation in the marine diatom Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2013, 97, 3625–3642. [Google Scholar] [CrossRef]
- Peng, X.; Liu, S.; Zhang, W.; Zhao, Y.; Chen, L.; Wang, H.; Liu, T. Triacylglycerol accumulation of Phaeodactylum tricornutum with different supply of inorganic carbon. J. Appl. Phycol. 2013, in press. [Google Scholar]
- Guckert, J.; Cooksey, K. Triglyceride accumulation and fatty acid profile changes in Chlorella (Chlorophyta) during high pH-induced cell inhibition. J. Phycol. 1990, 26, 72–79. [Google Scholar]
- Gardner, R.; Peters, P.; Peyton, B.; Cooksey, K. Medium pH and nitrate concentration effects on accumulation of triacylglycerol in two members of the Chlorophyta. J. Appl. Phycol. 2011, 26, 1005–1016. [Google Scholar]
- Toledo-Cervantes, A.; Morales, M.; Novelo, E.; Revah, S. Carbon dioxide fixation and lipid storage by Scenedesmus obtusiusculus. Bioresour. Technol. 2013, 130, 152–158. [Google Scholar]
- Jiang, Y.; Yoshida, T.; Quigg, A. Photosynthetic performance, lipid production and biomass composition in response to nitrogen limitation in marine microalgae. Plant Physiol. Biotechnol. 2012, 54, 70–77. [Google Scholar] [CrossRef]
- Guedes, A.C.; Meireles, L.A.; Amaro, H.M.; Malcata, F.X. Changes in lipid class and fatty acid composition of cultures of Pavlova lutheri, in response to light intensity. J. Am. Oil Chem. Soc. 2010, 87, 791–801. [Google Scholar]
- Guihéneuf, F.; Ulmann, L.; Tremblin, G.; Mimouni, V. Light-dependent utilization of two radiolabelled carbon sources, sodium bicarbonate and sodium acetate, and relationships with long chain polyunsaturated fatty acid synthesis in the microalga Pavlova lutheri (Haptophyta). Eur. J. Phycol. 2011, 46, 143–152. [Google Scholar] [CrossRef]
- Nimer, N.A.; Iglesias-Rodriguez, M.D.; Mmett, M.J. Bicarbonate utilization by marine phytoplankton species. J. Phycol. 1997, 33, 625–631. [Google Scholar]
- Tremblin, G.; Robert, J.M. Carbon fixation by the peculiar marine diatom Haslea ostrearia. Photosynthetica 2001, 39, 215–220. [Google Scholar] [CrossRef]
- Rech, M.; Morant-Manceau, A.; Tremblin, G. Carbon fixation and carbonic anhydrase activity in Haslea ostrearia (Bacillariophyceae) in relation to growth irradiance. Photosynthetica 2008, 46, 52–62. [Google Scholar]
- Reinfelder, J.R. Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annu. Rev. Mar. Sci. 2011, 3, 291–315. [Google Scholar] [CrossRef]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef]
- Chen, C.Y.; Durbin, E.G. Effects of pH on the growth and carbon uptake of marine phytoplankton. Mar. Ecol. Prog. Ser. 1994, 109, 83–94. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Leu, S.; Zarka, A.; Khozin-Goldberg, I.; Khalilov, I.; Boussiba, S. Cloning and molecular characterization of a novel acyl-CoA:diacylglycerol acyltransferase 1-like gene (PtDGAT1) from the diatom Phaeodactylum tricornutum. FEBS J. 2011, 278, 3651–3666. [Google Scholar] [CrossRef]
- Recht, L.; Zarka, A.; Boussiba, S. Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2012, 94, 1495–1503. [Google Scholar] [CrossRef]
- Sukenik, A.; Yamaguchi, Y. Alterations in lipid molecular species of the marine eustigmatophyte Nannochloropsis sp. J. Phycol. 1993, 29, 620–626. [Google Scholar]
- Brown, M.R.; Dunstan, G.A.; Norwood, S.J.; Miller, K.A. Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. J. Phycol. 1996, 32, 64–73. [Google Scholar]
- Tonon, T.; Harvey, D.; Larson, T.R.; Graham, I.A. Identification of a very long chain polyunsaturated fatty acid [Delta]4-desaturase from the microalga Pavlova lutheri. FEBS Lett. 2003, 553, 440–444. [Google Scholar]
- Fan, J.; Yan, C.; Andre, C.; Shanklin, J.; Schwender, J.; Xu, C. Oil accumulation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii. Plant Cell Physiol. 2012, 53, 1380–1390. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-K.; Cho, D.-H.; Ko, S.-R.; Oh, H.-M.; Kim, H.-S. Lipid droplet synthesis is limited by acetate availability in starchless mutant of Chlamydomonas reinhardtii. FEBS Lett. 2013, 587, 370–377. [Google Scholar]
- Cooksey, K.E.; Guckert, J.B.; Williams, S.A.; Callis, P.R. Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J. Microbiol. Methods 1987, 6, 333–345. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, B.-D.; Oh, H.-M. Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol. Tech. 1998, 12, 553–556. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.W.; Song, L.R.; Sommerfeld, M.; Hu, Q. A high throughput Nile Red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef]
- Tatsuzawa, H.; Takizawa, E. Changes in lipid and fatty acid composition of Pavlova lutheri. Phytochemistry 1995, 40, 397–400. [Google Scholar] [CrossRef]
- Culture Collection of Algae and Protozoa. Available online: http://www.ccap.ac.uk/ (accessed on 11 September 2013).
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- H2Ocean Salt Analysis. Available online: http://www.theaquariumsolution.com/files/H2Ocean%20Salt%20Analysis%20update_3.pdf (accessed on 11 September 2013).
- Collos, Y.; Mornet, F.; Sciandra, A.; Waser, N.; Larson, A.; Harrison, P.J. An optical method for the rapid measurement of micromolar concentrations of nitrate in marine phytoplankton cultures. J. Appl. Phycol. 1999, 11, 179–184. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Hu, G.; Dauvillee, D.; Sommerfeld, M.; Ball, S.; Hu, Q. Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyper-accumulates triacylglycerol. Metab. Eng. 2010, 12, 387–391. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guihéneuf, F.; Stengel, D.B. LC-PUFA-Enriched Oil Production by Microalgae: Accumulation of Lipid and Triacylglycerols Containing n-3 LC-PUFA Is Triggered by Nitrogen Limitation and Inorganic Carbon Availability in the Marine Haptophyte Pavlova lutheri. Mar. Drugs 2013, 11, 4246-4266. https://doi.org/10.3390/md11114246
Guihéneuf F, Stengel DB. LC-PUFA-Enriched Oil Production by Microalgae: Accumulation of Lipid and Triacylglycerols Containing n-3 LC-PUFA Is Triggered by Nitrogen Limitation and Inorganic Carbon Availability in the Marine Haptophyte Pavlova lutheri. Marine Drugs. 2013; 11(11):4246-4266. https://doi.org/10.3390/md11114246
Chicago/Turabian StyleGuihéneuf, Freddy, and Dagmar B. Stengel. 2013. "LC-PUFA-Enriched Oil Production by Microalgae: Accumulation of Lipid and Triacylglycerols Containing n-3 LC-PUFA Is Triggered by Nitrogen Limitation and Inorganic Carbon Availability in the Marine Haptophyte Pavlova lutheri" Marine Drugs 11, no. 11: 4246-4266. https://doi.org/10.3390/md11114246