Ophiobolin-O Reverses Adriamycin Resistance via Cell Cycle Arrest and Apoptosis Sensitization in Adriamycin-Resistant Human Breast Carcinoma (MCF-7/ADR) Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ophiobolin-O Reversed ADM Resistance
2.1.1. Ophiobolin-O Appeared to Reverse ADM Resistance in MCF-7/ADR Cells
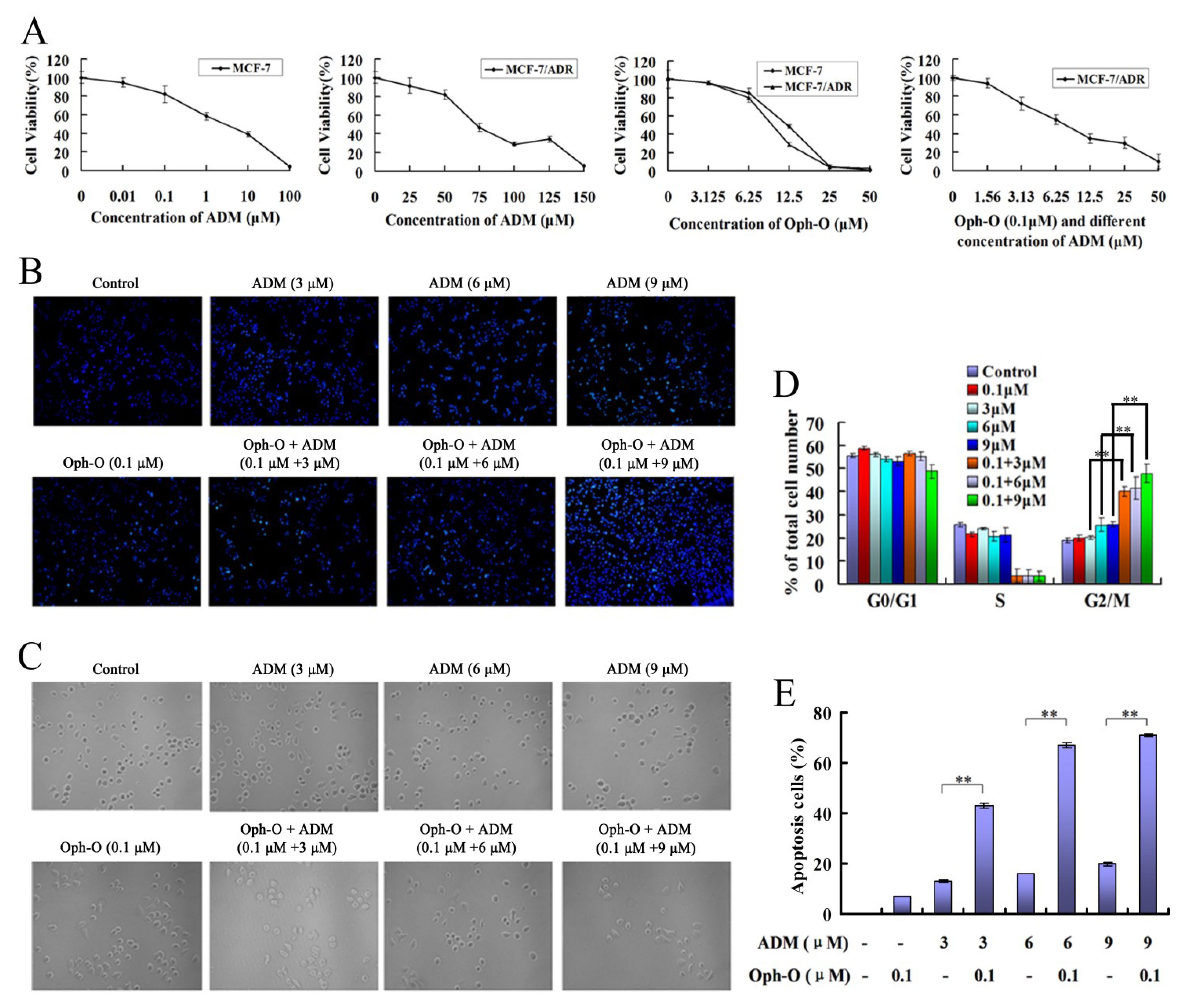
2.1.2. Nuclear Morphology Changes
2.1.3. Changes of Cell Count
2.1.4. Combination with Ophiobolin-O Caused G2/M Phase Arrest in MCF-7/ADR Cells
2.1.5. Ophiobolin-O Increased the Apoptosis Rate Induced by ADM in MCF-7/ADR Cells
2.2. ROS Mediated Ophiobolin-O Sensitized G2/M Arrest and Apoptosis of MCF-7/ADR Cells
2.2.1. The Combination Effect of Ophiobolin-O on Apoptotic and Cell Cycle Proteins in MCF-7/ADR Cells
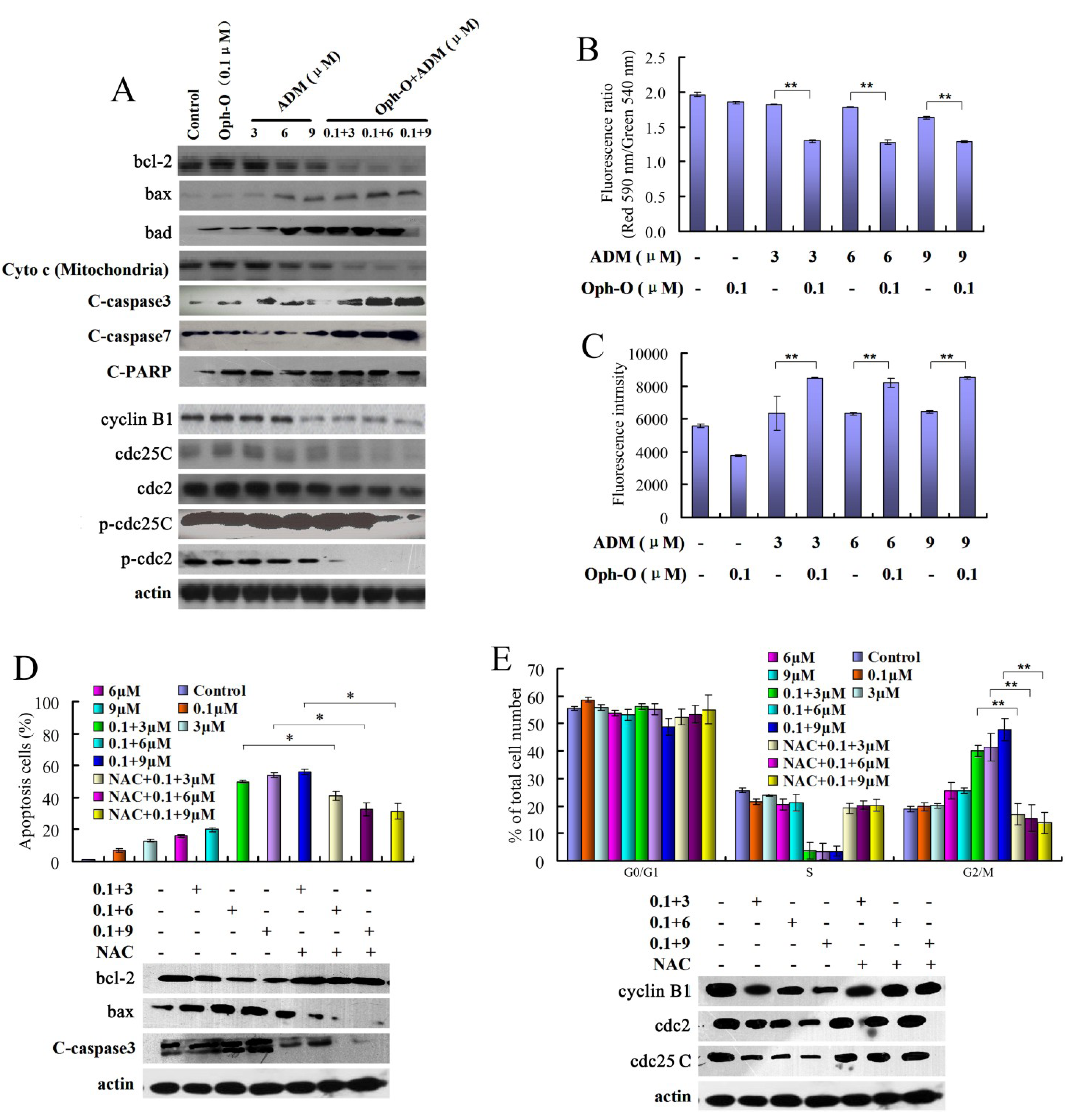
2.2.2. The Effects of Combination with Ophiobolin-O on Mitochondrial Membrane Potential of MCF-7/ADR Cells
2.2.3. ROS Was Involved in Reversal Effect of Ophiobolin-O
2.3. Ophiobolin-O Decreased the Expression of MDR1 and ADM Accumulation via Inhibiting the Activity of the MDR1 Gene Promoter
2.3.1. Ophiobolin-O Down-Regulated MDR1
2.3.2. Ophiobolin-O Inhibited Effect of P-Glycoprotein Function in MCF-7/ADR Cells
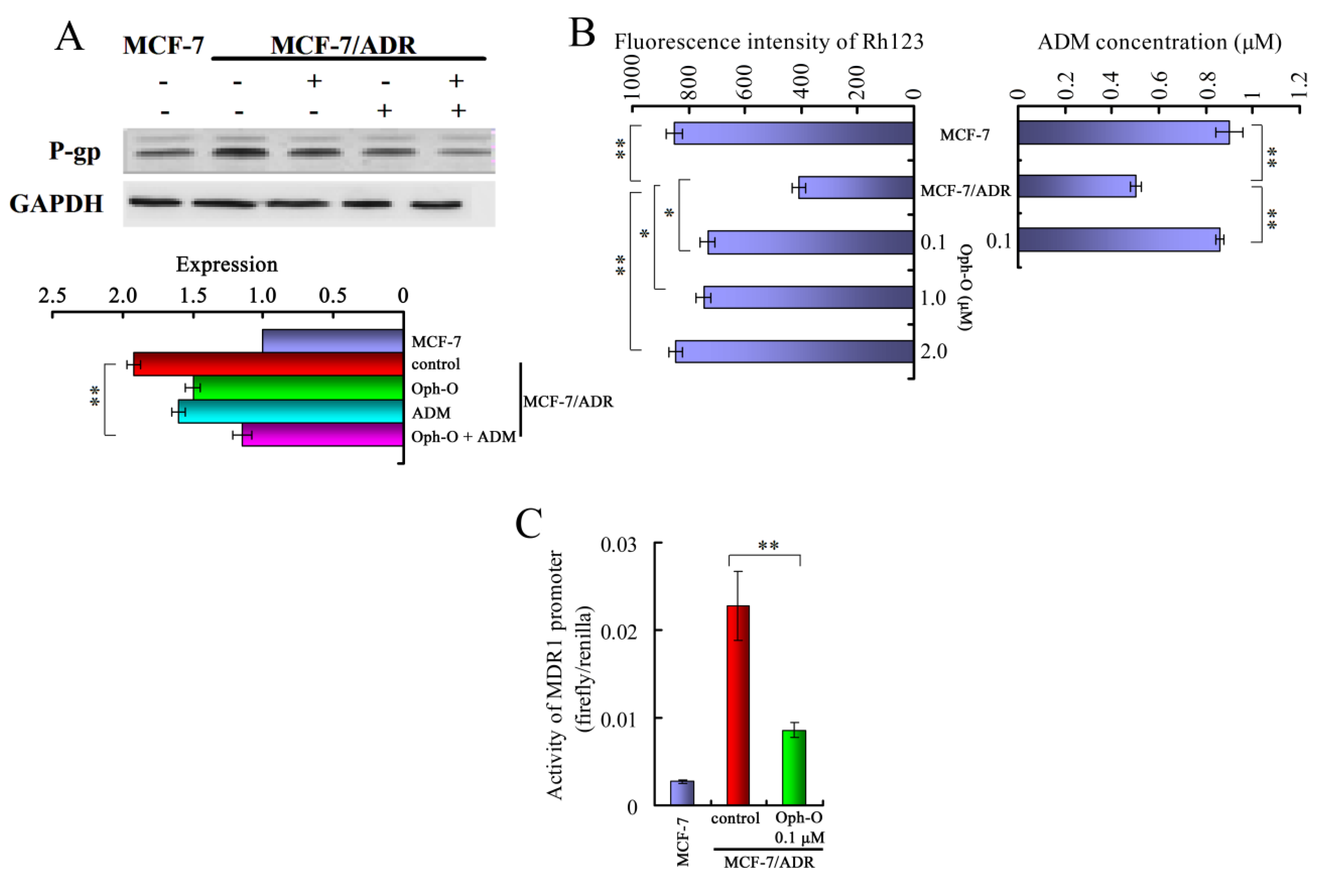
2.3.3. Ophiobolin-O Inhibited the Activity of MDR1 Gene Promoter
2.4. Inhibitory Effects on Tumor Growth of Combined Treatment with Ophiobolin-O and ADM in Nude Mice
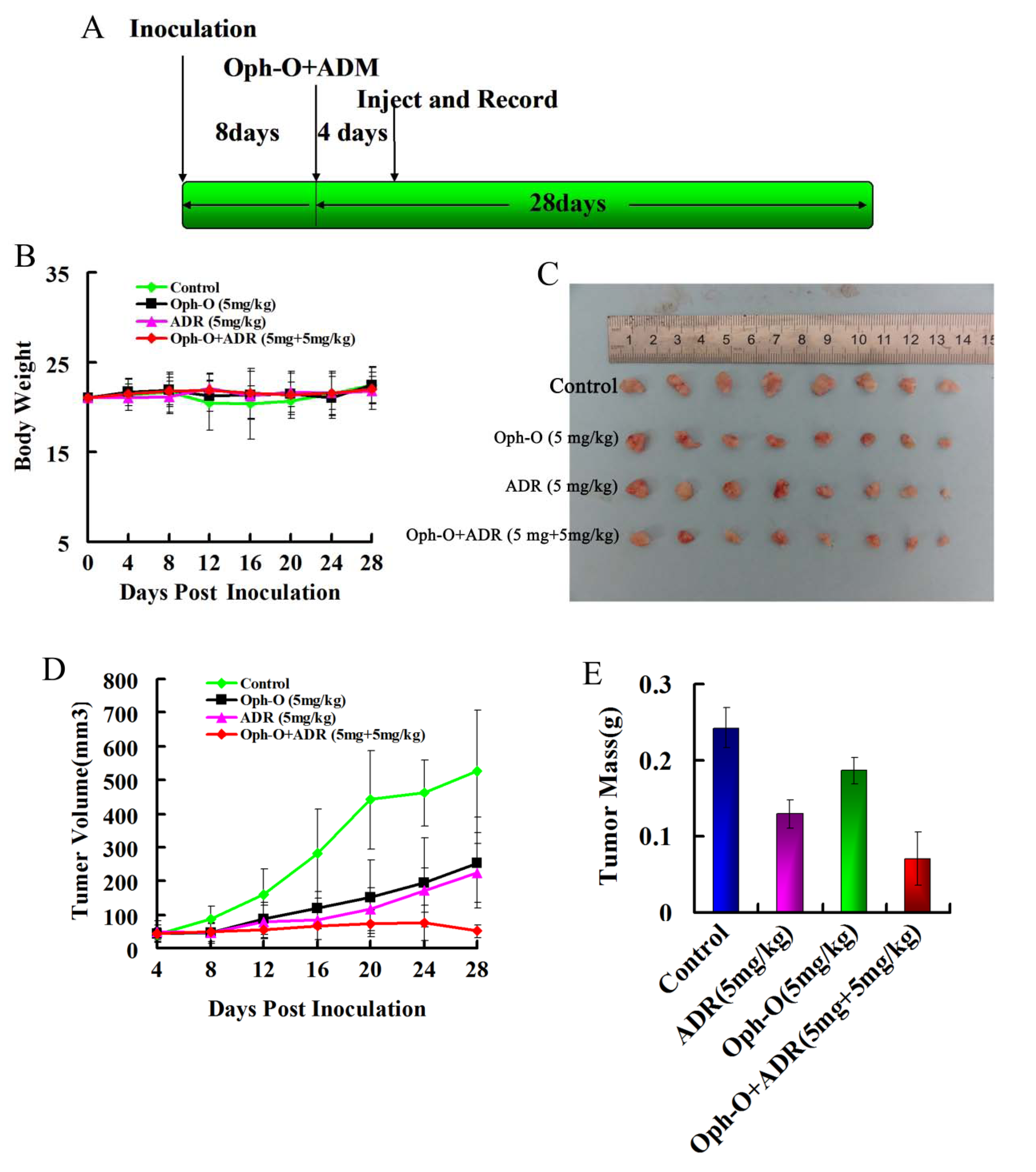
2.5. Discussion
3. Experimental Section
3.1. Compound and Cell Culture
3.2. Cell Viability Assay
3.3. Apoptosis and Cell Cycle Assay
3.4. Real-Time PCR
3.5. Western Blotting Analysis
3.6. Rhodamine 123 (Rh123) Efflux Studies
3.7. Intracellular Adriamycin Accumulation
3.8. MDR1 Promoter Activity by Vector Transient Transfection and Dual Luciferase Assay
3.9. Mouse Xenograft Model
4. Conclusions
Acknowledgments
References
- Zhuang, S.; Yan, Y.; Daubert, R.A.; Han, J.; Schnellmann, R.G. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am. J. Physiol. Renal. Physiol. 2007, 292, F440–F447. [Google Scholar]
- Zheng, A.; Kallio, A.; Härkönen, P. Tamoxifen-Induced rapid death of MCF-7 breast cancer cell is mediated via extracellularly signal-regulated kinase signaling and can be abrogated by estrogen. Endocrinology 2007, 148, 2764–2777. [Google Scholar] [CrossRef]
- Cole, S.P.; Bhardwaj, G.; Gerlach, J.H.; Mackie, J.E.; Grant, C.E.; Almquist, K.C.; Stewart, A.J.; Kurz, E.U.; Duncan, A.M.; Deeley, R.G. Over-Expression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992, 258, 1650–1654. [Google Scholar]
- Panaretakis, T.; Hjortsberg, L.; Tamm, K.P.; Björklund, A.C.; Joseph, B.; Grandér, D. Interferon alpha induces nucleus-independent apoptosis by activating extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase downstream of phosphatidylinositol 3-kinase and mammalian target of rapamycin. Mol. Biol. Cell 2008, 19, 41–50. [Google Scholar] [CrossRef]
- Yang, T.; Lu, Z.; Meng, L.; Wei, S.; Hong, K.; Zhu, W.; Huang, C. The novel agent Ophiobolin O induces apoptosis and cell cycly arrest of MCF-7 cells through activation of MAPK signaling pathways. Bioorg. Med. Chem. Lett. 2012, 22, 579–585. [Google Scholar] [CrossRef]
- Battle, T.E.; Arbiser, J.; Frank, D.A. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood 2005, 106, 690–697. [Google Scholar] [CrossRef]
- Hirano, T.; Gotoh, M.; Oka, K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci. 1994, 55, 1061–1069. [Google Scholar] [CrossRef]
- Fong, W.F.; Tse, A.K.; Poon, K.H.; Wang, C. Magnolol and honokiol enhance HL-60 human leukemia cell differentiation induced by 1, 25-dihydroxyvitamin D3 and retinoic acid. Int. J. Biochem. Cell Biol. 2005, 37, 427–441. [Google Scholar] [CrossRef]
- Bai, X.; Cerimele, F.; Ushio-Fukai, M.; Waqas, M.; Campbell, P.M.; Govindarajan, B.; Der, C.J.; Battle, T.; Frank, D.A.; Ye, K.; et al. Govindarajan, Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J. Biol. Chem. 2003, 278, 35501–35507. [Google Scholar] [CrossRef]
- Wang, T.; Chen, F.; Chen, Z.; Wu, Y.F.; Xu, X.L.; Zheng, S.; Hu, X. Honokiol induces apoptosis through p53-independent pathway in human colorectal cell line RKO. World J. Gastroenterol 2004, 10, 2205–2208. [Google Scholar]
- Chen, F.; Wang, T.; Wu, Y.F.; Gu, Y.; Xu, X.L.; Zheng, S.; Hu, X. Honokiol: A potent chemotherapy candidate for human colorectal carcinoma. World J. Gastroenterol 2004, 10, 3459–3463. [Google Scholar]
- Schiedlmeier, B.; Kühlcke, K.; Eckert, H.G.; Baum, C.; Zeller, W.J.; Fruehauf, S. Quantitative assessment of retroviral transfer of the human multidrug resistance 1 gene to human mobilized peripheral blood progenitor cells engrafted in nonobese diabetic/severe combined immunodeficient mice. Blood 2000, 95, 1237–1248. [Google Scholar]
- Sonneveld, P.; Wiemer, E. Inhibitors of multidrug resistance. Curr. Opin. Oncol. 1997, 9, 543–548. [Google Scholar] [CrossRef]
- Li, G.Y.; Liu, J.Z.; Zhang, B.; Wang, L.X.; Wang, C.B.; Chen, S.G. Cyclosporine diminishes multidrug resistance in K562/ADM cells and improves complete remission in patients with acute myeloid leukemia. Biomed. Pharmacother. 2009, 63, 566–570. [Google Scholar] [CrossRef]
- Limtrakul, P.; Anuchapreeda, S.; Buddhasukh, D. Modulation of human multidrug-resistance MDR-1 gene by natural curcuminoids. Biomed. Chromatogr. Cancer 2004, 4. [Google Scholar] [CrossRef]
- Chaudhary, P.M.; Roninson, I.B. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J. Natl. Cancer Inst. 1993, 85, 632–639. [Google Scholar] [CrossRef]
- Herzog, C.E.; Tsokos, M.; Bates, S.E.; Fojo, A.T. Increased mdr-1/P-glycoprotein expression after treatment of human colon carcinoma cells with P-glycoprotein antagonists. J. Biol. Chem. 1993, 268, 2946–2952. [Google Scholar]
- Hall, J.M.; McDonnell, D.P.; Korach, K.S. Allosteric regulation of estrogen receptor structure, function, and coactivator recruitment by different estrogen response elements. Mol. Endocrinol. 2002, 16, 469–486. [Google Scholar] [CrossRef]
- Klinge, C.M.; Jernigan, S.C.; Mattingly, K.A.; Risinger, K.E.; Zhang, J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors. J. Mol. Endocrinol. 2004, 33, 387–410. [Google Scholar] [CrossRef]
- Ponce, de León V.; Barrera-Rodríguez, R. Changes in P-glycoprotein activity are mediated by the growth of a tumorcell line as multicellular spheroids. Cancer Cell Int. 2005, 5. [Google Scholar] [CrossRef] [Green Version]
- Takahashi-Yanaga, F.; Sasaguri, T. GSK-3beta regulates cyclin D1 expression: A new target for chemotherapy. Cell Signal. 2008, 20, 581–589. [Google Scholar] [CrossRef]
- Ling, V. Multidrug resistance: Molecular mechanisms and clinical relevance. Cancer Chemother. Pharmacol. 1997, 40, S3–S8. [Google Scholar] [CrossRef]
- Pettit, R.K.; Pettit, G.R.; Xu, J.P.; Weber, C.A.; Richert, L.A. Isolation of human cancer cell growth inhibitory, antimicrobial lateritin from a mixed fungal culture. Planta Med. 2010, 76, 500–501. [Google Scholar] [CrossRef]
- Pyo, M.K.; Lee, Y.; Yun-Choi, H.S. Anti-Platelet effect of the constituents isolated from the barks and fruits of magnolia obovata. Arch. Pharm. Res. 2002, 25, 325–328. [Google Scholar] [CrossRef]
- Park, E.J.; Zhao, Y.Z.; Na, M.; Bae, K.; Kim, Y.H.; Lee, B.H.; Sohn, D.H. Protective effects of honokiol and magnolol on tertiary butyl hydroperoxide-or d-galactosamine-induced toxicity in rat primary hepatocytes. Planta Med. 2003, 69, 33–37. [Google Scholar] [CrossRef]
- Chen, L.M.; Liang, Y.J.; Ruan, J.W.; Ding, Y.; Wang, X.W.; Shi, Z.; Gu, L.Q.; Yang, X.P.; Fu, L.W. Reversal of P-gp mediated multidrug resistance in vitro and in vivo by FG020318. J. Pharm. Pharmacol. 2004, 56, 1061–1066. [Google Scholar] [CrossRef]
- Kuribara, H.; Stavinoha, W.B.; Maruyama, Y. A putative anxiolytic agent extracted from magnolia bark, has no diazepam-like side-effects in mice. J. Pharm. Pharmacol. 1999, 51, 97–103. [Google Scholar]
- Shin, T.Y.; Kim, D.K.; Chae, B.S.; Lee, E.J. Antiallergic action of magnolia officinalis on immediate hypersensitivity reaction. Arch. Pharm. Res. 2001, 24, 249–255. [Google Scholar] [CrossRef]
- Chang, B.; Lee, Y.; Ku, Y.; Bae, K.; Chung, C. Antimicrobial activity of magnolol and honokiol against periodontopathic microorganisms. Planta Med. 1998, 64, 367–369. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, W.; Lv, C.; Zhu, T.; Yang, X.; Wei, S.; Sun, J.; Hong, K.; Zhu, W.; Huang, C. Ophiobolin-O Reverses Adriamycin Resistance via Cell Cycle Arrest and Apoptosis Sensitization in Adriamycin-Resistant Human Breast Carcinoma (MCF-7/ADR) Cells. Mar. Drugs 2013, 11, 4570-4584. https://doi.org/10.3390/md11114570
Sun W, Lv C, Zhu T, Yang X, Wei S, Sun J, Hong K, Zhu W, Huang C. Ophiobolin-O Reverses Adriamycin Resistance via Cell Cycle Arrest and Apoptosis Sensitization in Adriamycin-Resistant Human Breast Carcinoma (MCF-7/ADR) Cells. Marine Drugs. 2013; 11(11):4570-4584. https://doi.org/10.3390/md11114570
Chicago/Turabian StyleSun, Wenxia, Cuiting Lv, Tonghan Zhu, Xue Yang, Shanjian Wei, Jieyin Sun, Kui Hong, Weiming Zhu, and Caiguo Huang. 2013. "Ophiobolin-O Reverses Adriamycin Resistance via Cell Cycle Arrest and Apoptosis Sensitization in Adriamycin-Resistant Human Breast Carcinoma (MCF-7/ADR) Cells" Marine Drugs 11, no. 11: 4570-4584. https://doi.org/10.3390/md11114570



