The Protection of Polysaccharide from the Brown Seaweed Sargassum graminifolium against Ethylene Glycol-Induced Mitochondrial Damage
Abstract
:Abbreviations
| SGP | polysaccharide from Sargassum graminifolium |
| Ox | oxalate |
| CaOx | calcium oxalate |
| SDH | succinate dehydrogenase |
| MDA | malondialdehyde |
| ROS | reactive oxygen species |
| EG | ethylene glycol |
| AC | ammonium chloride |
| ATP | adenosine triphosphate |
| SOD | superoxide dismutase |
| GSH-PX | glutathione peroxidase |
| CAT | catalase |
1. Introduction
2. Results and Discussion
2.1. Characterization of SGP
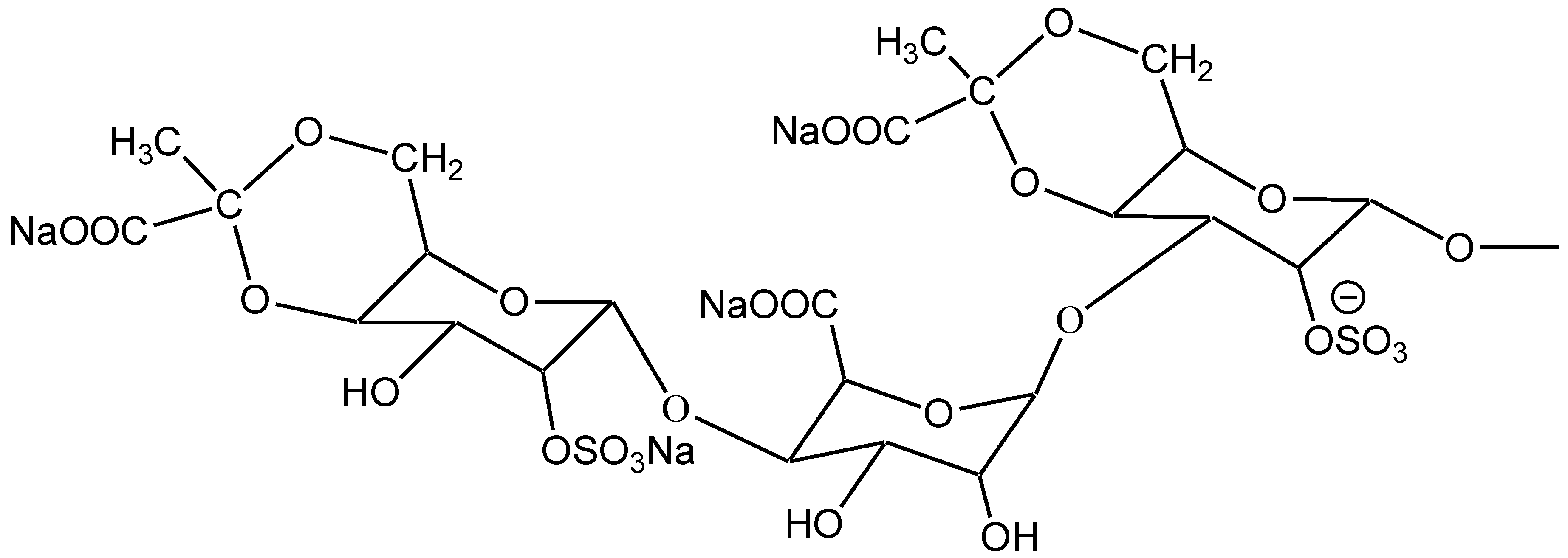
2.2. Effects of SGP on Mitochondrial Lipid Peroxidation
2.3. Effects of SGP on Mitochondrial Swelling
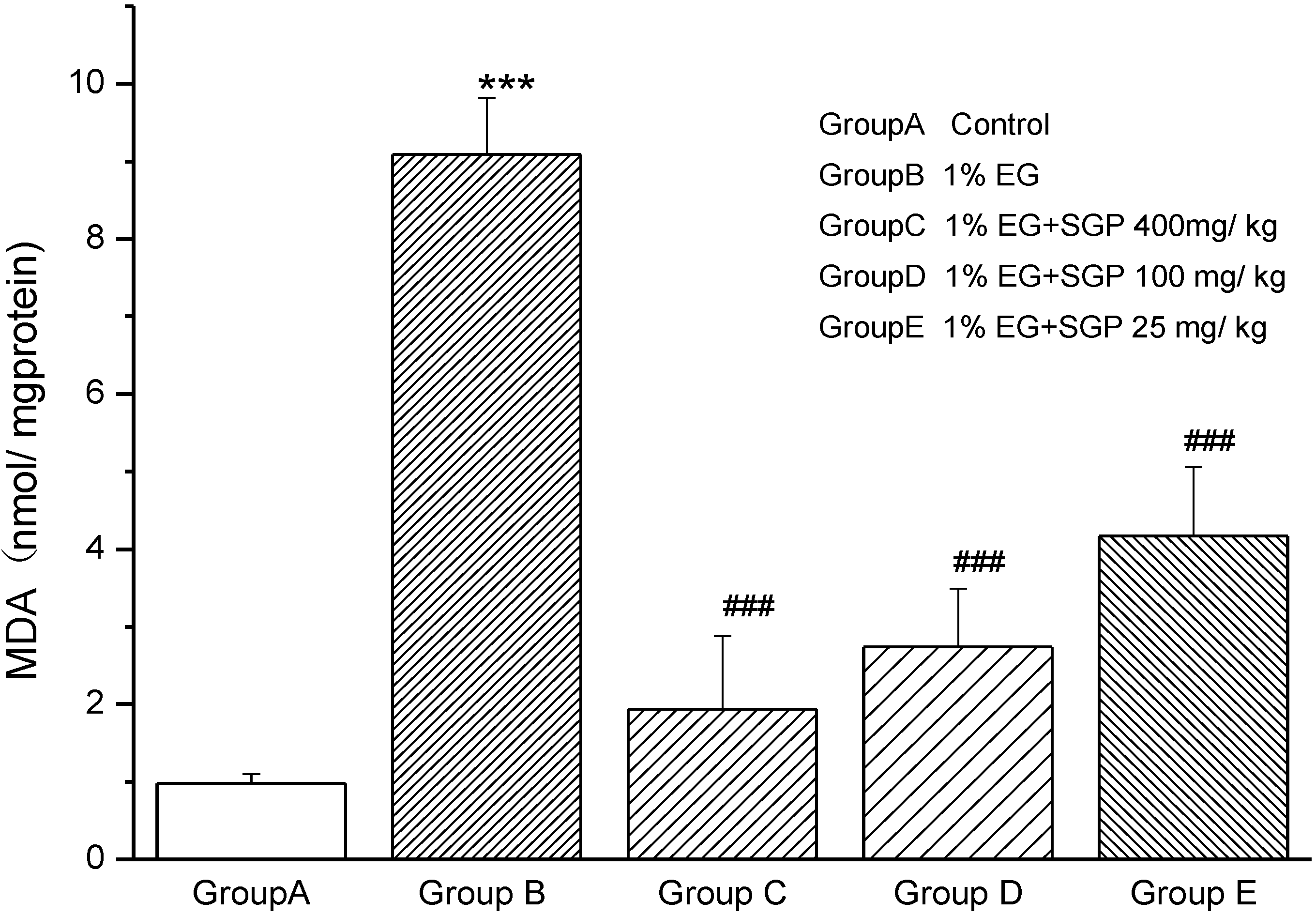
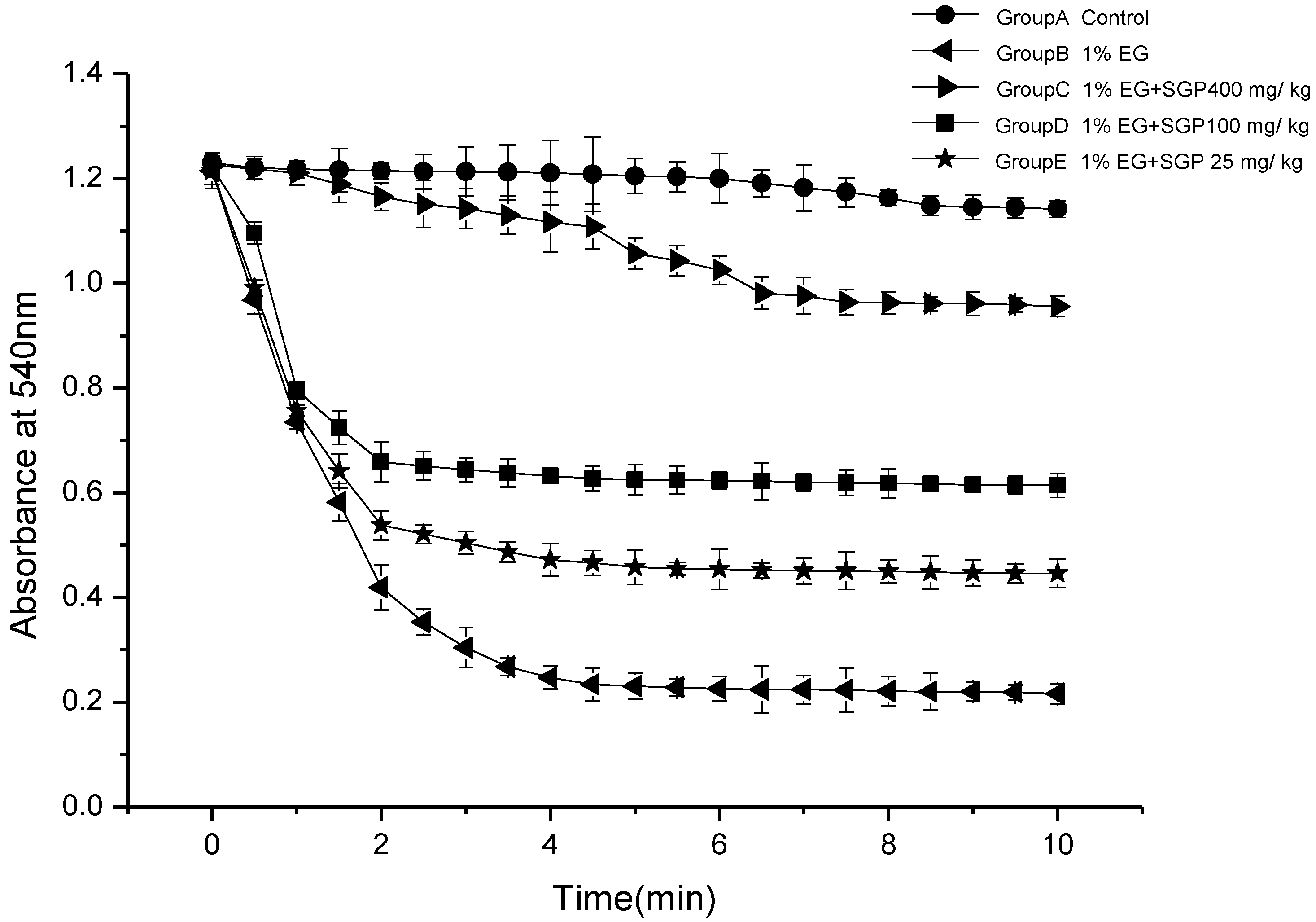
2.4. Effects of SGP on SDH Content of Mitochondrial
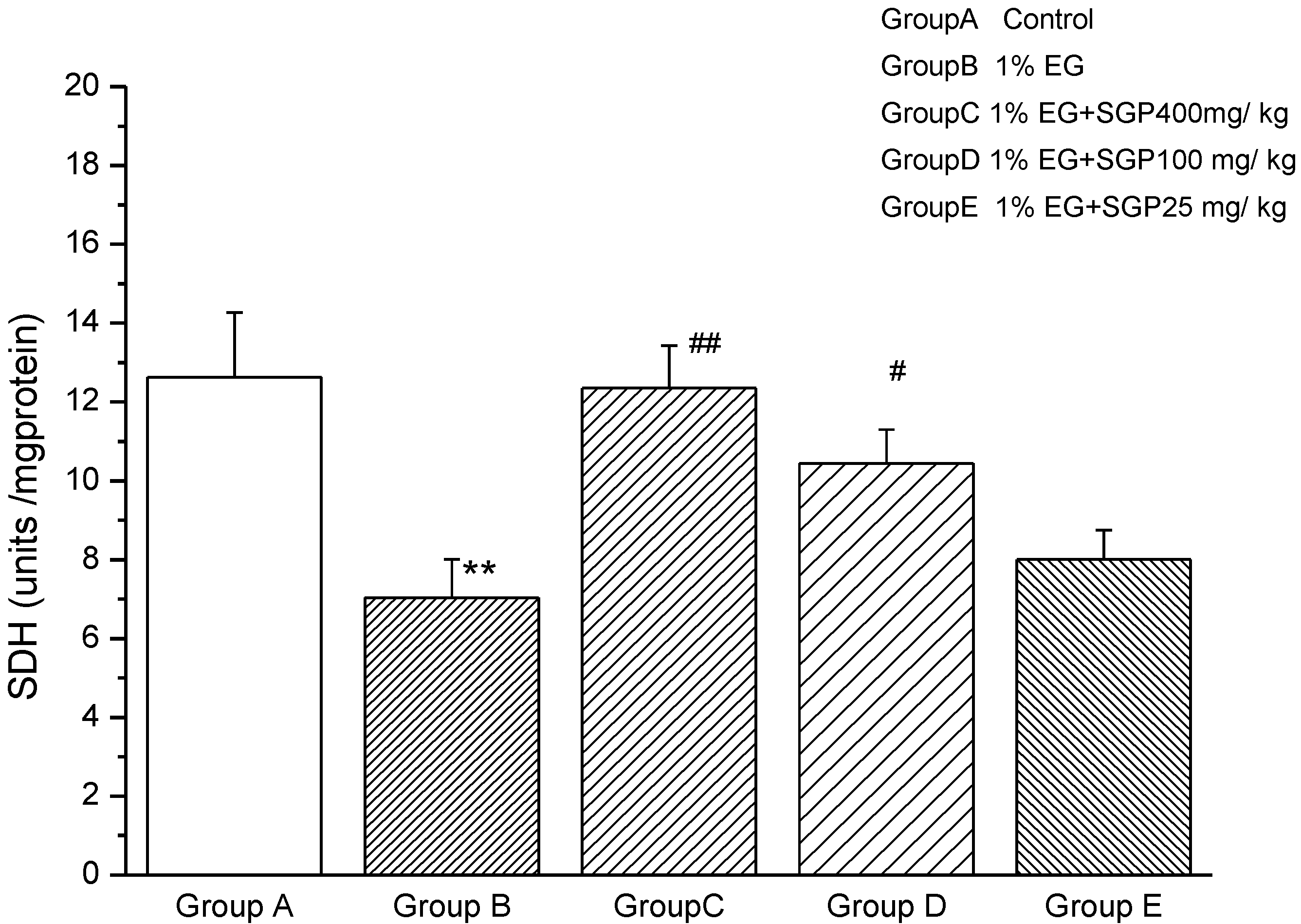
2.5. Effects of SGP on Mitochondrial ATPases
| Parameters | Group A | Group B | Group C | Group D | Group E |
|---|---|---|---|---|---|
| Na+/K+-ATPases | 3.05 ± 0.23 | 1.36 ± 0.07 *** | 2.97 ± 0.15 ## | 2.32 ± 0.29 # | 1.47 ± 0.18 |
| Ca2+-ATPases | 2.25 ± 0.19 | 1.05 ± 0.03 ** | 2.18 ± 0.16 ## | 2.01 ± 0.21 ## | 1.96 ± 0.13 # |
| Mg2+-ATPases | 2.88 ± 0.17 | 0.90 ± 0.04 *** | 2.69 ± 0.0 ### | 2.12 ± 0.11 ## | 1.24 ± 0.12 |
2.6. Effects of SGP on Mitochondrial Antioxidant Enzymes
| Parameters | Group A | Group B | Group C | Group D | Group E |
|---|---|---|---|---|---|
| SOD (U/mg prot) | 58.22 ± 8.33 | 36.44 ± 4.94 ** | 53.46 ± 3.58 # | 43.33 ± 2.0 # | 36.62 ± 2.66 |
| GSH-Px (U/mg prot) | 35.24 ± 2.13 | 15.72 ± 1.47 ** | 32.76 ± 3.05 ## | 25.29 ± 2.58 # | 18.55 ± 1.72 # |
| Catalate (U/mg prot) | 31.58 ± 0.94 | 15.15 ± 1.17 ** | 18.76 ± 0.93 # | 21.43 ± 0.67 # | 27.07 ± 0.73 # |
2.7. Discussion
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Animals
3.4. Ethylene Glycol-Induced Mitochondrial Damage in Rats
3.5. Preparation of Mitochondria
3.6. Mitochondrial Lipid Peroxidation
3.7. Measurement of Mitochondrial Swelling
3.8. Assay of the Activity of the Succinate Dehydrogenase (SDH)
3.9. Assay of activities of ATPases
3.10. Mitochondrial Antioxidant Enzymes
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int. J. Biol. Macromol. 1995, 5, 293–297. [Google Scholar]
- Karnjanapratum, S.; You, S. Molecular characteristics of sulfated polysaccharides from Monostroma nitidum and their in vitro anticancer and immunomodulatory activities. Int. J. Biol. Macromol. 2010, 2, 311–318. [Google Scholar]
- Rajeswari, A.; Varalakshmi, P. Low molecular weight heparin protection against oxalate-induced oxidative renal insult. Clin. Chim. Acta 2006, 1, 108–114. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 1, 14–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Zhang, H.; Niu, X.; Li, P. Preparation of the different derivatives of the low-molecular-weight porphyran from Porphyra haitanensis and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2009, 1, 22–26. [Google Scholar]
- Kardošová, A.; Machová, E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia 2006, 77, 367–373. [Google Scholar] [CrossRef]
- Borghi, L.; Meschi, T.; Guerra, A.; Bergamaschi, E.; Mutti, A.; Novarini, A. Effects of urinary macromolecules on the nucleation of calcium oxalate in idiopathic stone formers and healthy controls. Clin. Chim. Acta 1995, 1, 1–11. [Google Scholar]
- Edyvane, K.A.; Hibberd, C.M.; Harnett, R.M.; Marshall, V.R.; Ryall, R.L. Macromolecules inhibit calcium oxalate crystal growth and aggregation in whole human urine. Clin. Chim. Acta 1987, 3, 329–338. [Google Scholar]
- Liu, J.; Wang, T.; Chen, J.; Wang, S.; Ye, Z. Decreased inhibitory activity of prothrombin to calcium oxalate crystallization by specific chemical modification of its gamma-carboxyglutamic acid residues. Urology 2006, 1, 201–203. [Google Scholar]
- Hackett, R.L.; Shevock, P.N.; Khan, S.R. Madin-Darby canine kidney cells are injured by exposure to oxalate and to calcium oxalate crystals. Urol. Res. 1994, 22, 197. [Google Scholar] [CrossRef]
- Koul, H.; Kenington, L.; Honeyman, T.; Jonassen, J.; Menon, M.; Scheid, C.R. Activation of the c-myc gene mediates the mitogenic effects of Ox in LLC-PK1 cells, a line of renal epithelial cells. Kidney Int. 1996, 50, 1525. [Google Scholar] [CrossRef]
- Bashir, S.; Gilani, A.H. Antiurolithic effect of Bergenia ligulata rhizome: An explanation of the underlying mechanisms. J. Ethnopharmacol. 2009, 1, 106–116. [Google Scholar] [CrossRef]
- Niimi, K.; Yasui, T.; Hirose, M.; Hamamoto, S.; Itoh, Y.; Okada, A.; Kubota, Y.; Kojima, Y.; Tozawa, K.; Sasaki, S.; et al. Mitochondrial permeability transition pore opening induces the initial process of renal calcium crystallization. Free Radic. Biol. Med. 2012, 52, 1207–1217. [Google Scholar] [CrossRef]
- Cao, L.C.; Honeyman, T.W.; Cooney, R.; Kennington, L.; Scheid, C.R.; Jonassen, J.A. Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int. 2004, 66, 1890–1900. [Google Scholar]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta Rev. Biomembr. 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Rajesh, N.G.; Varalakshmi, P. Mitochondrial dysfunction in an animal model of hyperoxaluria: A prophylactic approach with fucoidan. Eur. J. Pharmacol. 2008, 579, 330–336. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Zhang, H.; Niu, X.Z. Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica. Int. J. Biol. Macromol. 2010, 47, 126–131. [Google Scholar] [CrossRef]
- Samee, H.; Li, Z.X.; Lin, H.; Khalid, J.; Wang, B.P. In vivo study of antiallergenicity of ethanol extracts from Sargassum tenerrimum, Sargassum cervicorne and Sargassum graminifolium turn. Eur. Food Res. Technol. 2009, 229, 435–441. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Wu, W.-H.; Lan, M.-B. Antioxidant properties of polysaccharide from the brown Seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Mar. Drugs 2012, 10, 119–130. [Google Scholar] [CrossRef]
- Bubber, P.; Ke, Z.-J.; Gibson, G.E. Tricarboxylic acid cycle enzymes following thiamine deficiency. Neurochem. Int. 2004, 45, 1021–1028. [Google Scholar] [CrossRef]
- Küçükkurt, I.; Ince, S.; Keles, H.; Akkol, E.K.; Avci, G.; Yesilada, E.; Bacak, E. Beneficial effects of Aesculus hippocastanum L. seed extract on the body’s own antioxidant defense system on subacute administration. J. Ethnopharmacol. 2010, 129, 18–22. [Google Scholar] [CrossRef]
- Srinivasan, S.; Pragasam, V.; Jenita, X.; Kalaiselvi, P.; Muthu, V.; Varalakshmi, P. Oxidative stress in urogenital tuberculosis patients: A predisposing factor for renal stone formation amelioration by vitamin E supplementation. Clin. Chim. Acta 2004, 350, 57–63. [Google Scholar] [CrossRef]
- Muthukumar, A.; Selvam, R. Role of glutathione on renal mitochondrial status in Hyperoxaluria. Mol. Cell. Biochem. 1998, 185, 77–84. [Google Scholar] [CrossRef]
- Chaiyarit, S.; Thongboonkerd, V. Changes in mitochondrial proteome of renal tubular cells induced by calcium oxalate monohydrate crystal adhesion and internalization are related to mitochondrial dysfunction. J. Proteome Res. 2012, 11, 3269–3280. [Google Scholar] [CrossRef]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Varalakshmi, P. Beneficial role of sulfated polysaccharides from edible seaweed Fucus vesiculosus in experimental hyperoxaluria. Food Chem. 2007, 100, 1552–1559. [Google Scholar] [CrossRef]
- Bayir, Y.; Halici, Z.; Keles, M.S.; Colak, S.; Cakir, A.; Kaya, Y.; Akçay, F. Helichrysum plicatum DC. subsp. plicatum extract as a preventive agent in experimentally induced urolithiasis model. J. Ethnopharmacol. 2011, 138, 408–414. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, C.-Y.; Kong, T.; Wu, W.-H.; Lan, M.-B. The Protection of Polysaccharide from the Brown Seaweed Sargassum graminifolium against Ethylene Glycol-Induced Mitochondrial Damage. Mar. Drugs 2013, 11, 870-880. https://doi.org/10.3390/md11030870
Zhang C-Y, Kong T, Wu W-H, Lan M-B. The Protection of Polysaccharide from the Brown Seaweed Sargassum graminifolium against Ethylene Glycol-Induced Mitochondrial Damage. Marine Drugs. 2013; 11(3):870-880. https://doi.org/10.3390/md11030870
Chicago/Turabian StyleZhang, Chao-Yan, Ting Kong, Wen-Hui Wu, and Min-Bo Lan. 2013. "The Protection of Polysaccharide from the Brown Seaweed Sargassum graminifolium against Ethylene Glycol-Induced Mitochondrial Damage" Marine Drugs 11, no. 3: 870-880. https://doi.org/10.3390/md11030870




