Antinociceptive Activity of Stephanolepis hispidus Skin Aqueous Extract Depends Partly on Opioid System Activation
Abstract
:1. Introduction
2. Results and Discussion
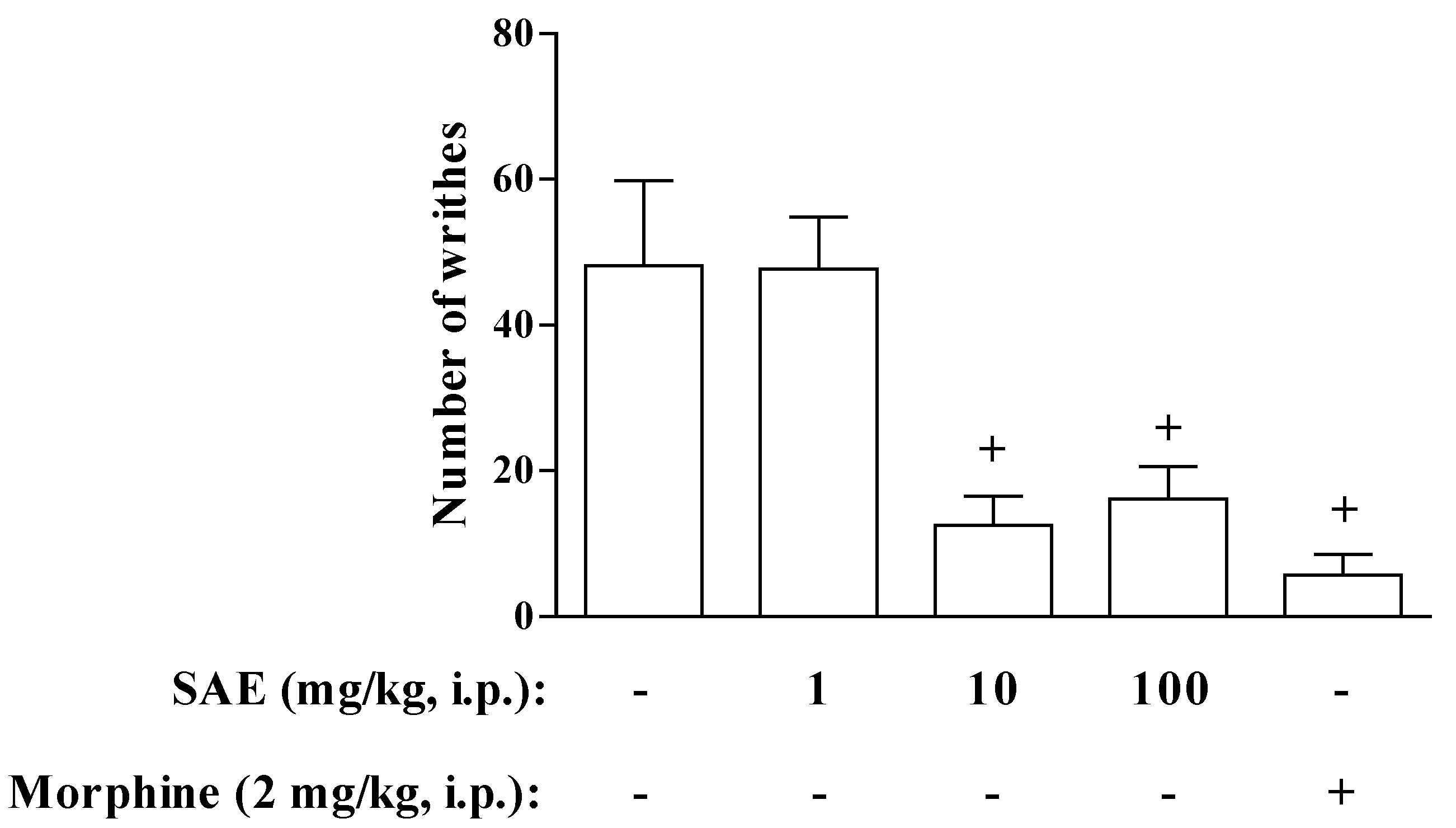
| Treatment | (mg/kg) | Number of writhes |
|---|---|---|
| SAE | - | 40 ± 4 |
| 10 | 40 ± 8 | |
| 100 | 35 ± 3 | |
| 1000 | 30 ± 4 |



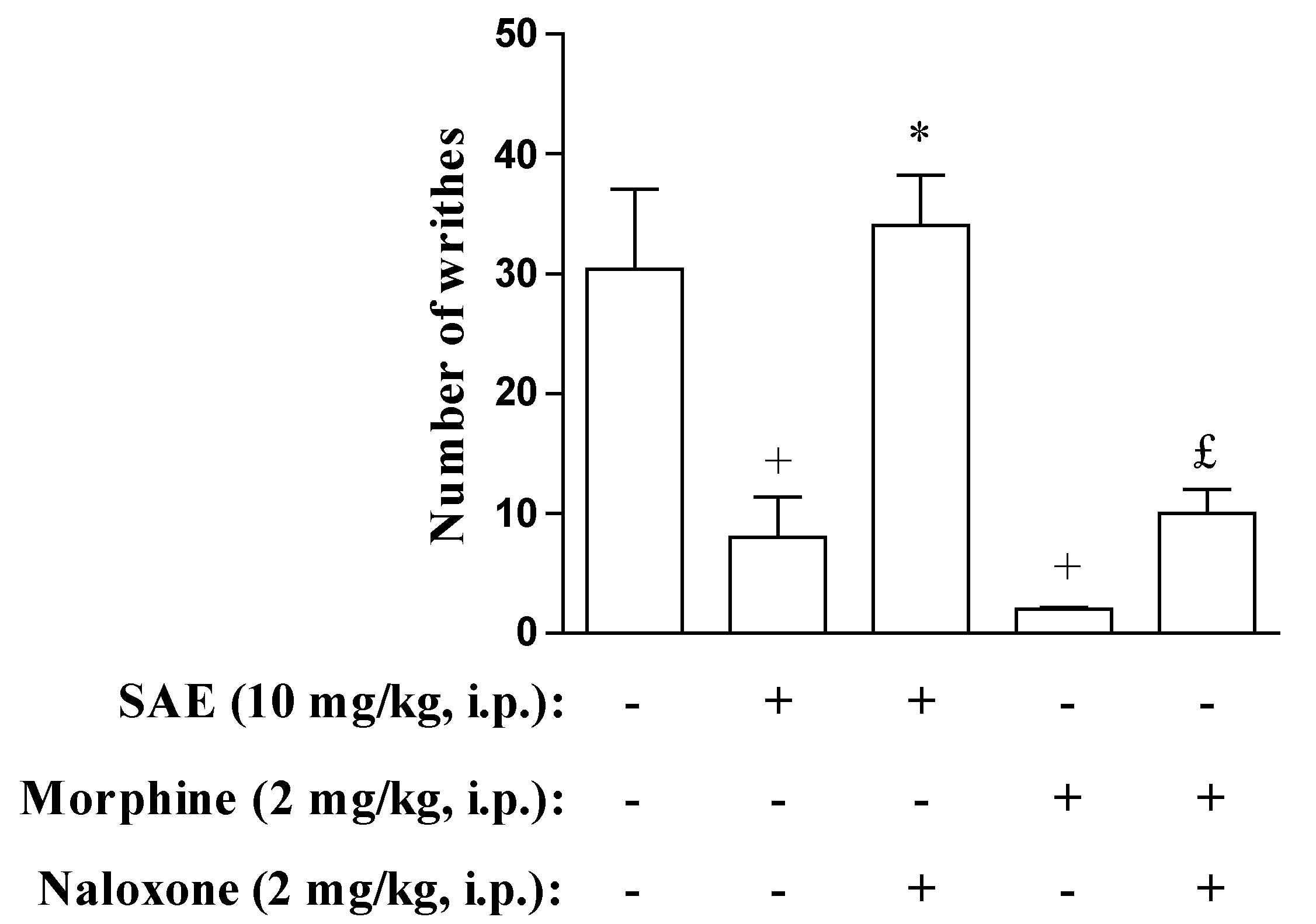
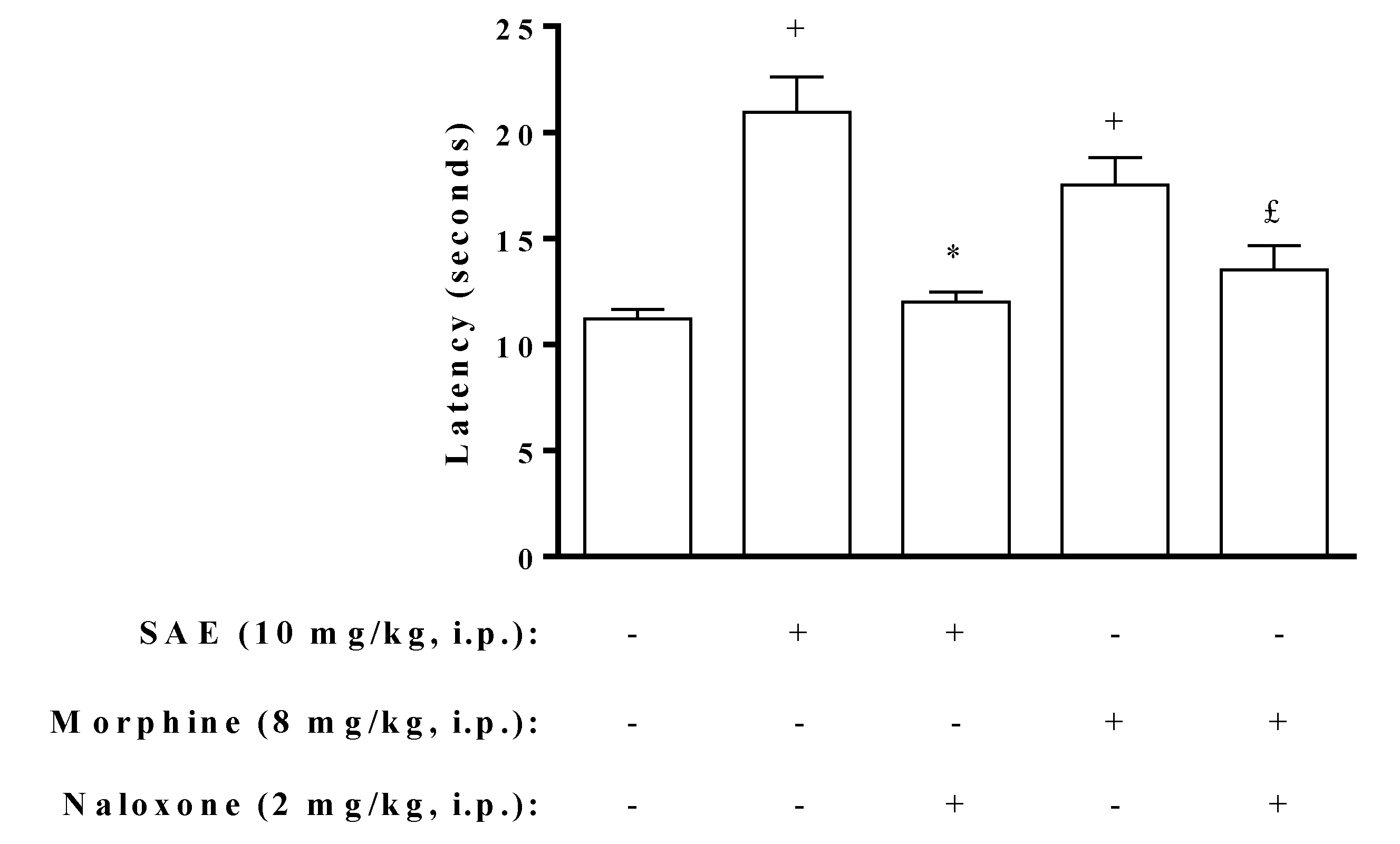
| Treatments (μg/mL) | Levels of DNA damage (%) | DNA damage score Total arbitrary units | Cytotoxicity (% of death cells) | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| SAE | - | 94.00 ± 2.00 | 5.00 ± 1.50 | 0.50 ± 0.00 | 0.50 ± 0.50 | 15.00 ± 6.00 | 0.00 |
| 0.05 | 97.00 ± 0.00 | 2.75 ± 0.25 | 0.25 ± 0.25 | 0.00 ± 0.00 | 6.50 ± 0.50 | 0.00 | |
| 0.5 | 97.00 ± 0.50 | 2.25 ± 0.75 | 0.25 ± 0.25 | 0.50 ± 0.00 | 8.50 ± 0.50 | 0.00 | |
| 5 | 95.75 ± 0.25 | 3.75 ± 0.75 | 0.25 ± 0.25 | 0.25 ± 0.25 | 10.00 ± 1.00 | 0.00 | |
| 50 | 95.75 ± 0.75 | 4.00 ± 1.00 | 0.25 ± 0.25 | 0.00 ± 0.00 | 9.00 ± 1.00 | 0.00 | |
| 500 | 94.00 ± 2.50 | 6.00 ± 2.50 | 0.00 ± 0.00 | 0.00 ± 0.00 | 12.00 ± 5.00 | 0.00 | |
| 1000 | 93.25 ± 3.25 | 6.50 ± 3.00 | 0.00 ± 0.00 | 0.25 ± 0.25 | 14.50 ± 7.50 | 0.50 | |
| 2500 | 92.50 ± 1.50 | 6.75 ± 1.75 | 0.75 ± 0.25 | 0.00 ± 000 | 16.50 ± 2.50 | 0.00 | |
| 5000 | 91.50 ± 3.00 | 8.50 ± 3.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 17.00 ± 6.00 | 0.50 | |
| MMS | 0.00 ± 0.00 | 6.75 ± 6.75 | 7.75 ± 4.25 | 85.50 ± 11.0 | 557.50 ± 35.50 * | - | |
3. Experimental Section
3.1. Biological Materials
3.2. Preparation of Extract
3.3. Animals
3.4. Abdominal Writhing Test
3.5. Hot Plate Test
3.6. Hyperalgesia
3.7. Tail-Flick Test
3.8. Endotoxin Evaluation
3.9. Toxicological Tests
3.9.1. Cytotoxicity
3.9.2. Comet Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
References
- De Souza, E.T.; de Lira, D.P.; de Queiroz, A.C.; da Silva, D.J.; de Aquino, A.B.; Mella, E.A.; Lorenzo, V.P.; de Miranda, G.E.; de Araujo-Junior, J.X.; Chaves, M.C.; et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar]
- Queiroz, T.M.; Machado, N.T.; Furtado, F.F.; Oliveira-Filho, A.A.; Alustau, M.C.; Figueiredo, C.S.; Miranda, G.E.; Barbosa-Filho, J.M.; Braga, V.A.; Medeiros, I.A. Vasorelaxation, induced by Dictyota pulchella (Dictyotaceae), a brown alga, is mediated via inhibition of calcium influx in rats. Mar. Drugs 2011, 9, 2075–2088. [Google Scholar]
- Jha, R.; Zi-Rong, X. Biomedical compounds from marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar]
- Kerr, R.G.; Kerr, S.S. Marine natural products as therapeutic agents. Expert Opin. Ther. Pat. 1999, 9, 1207–1222. [Google Scholar]
- Koopmans, M.; Martens, D.; Wijffels, R.H. Towards commercial production of sponge medicines. Mar. Drugs 2009, 7, 787–802. [Google Scholar]
- Mayer, A.M.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef]
- Nelson, J.S. Fishes of the World, 4th ed; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 485–538. [Google Scholar]
- Scott, W.B.; Scott, M.G. Atlantic Fishes of Canada, 1st ed; Canadian Bulletin of Fisheries and Aquatic Sciences: Toronto, Canada, 1988; pp. 645–704. [Google Scholar]
- Figueiredo, J.L. Peixes da Zona Econômica Exclusiva da Região Sudeste-Sul do Brasil: Levantamento Com Rede de Meia Água, 1st ed; da Universidade de São Paulo: São Paulo, Brazil, 2002; p. 242. [Google Scholar]
- Harmelin-Vivien, M.L.; Quéro, J.C. Monacanthidae. In Clofeta: Check-List of the Fishes of the Eastern Tropical Atlantic, 1st; Post, J.C., Saldanha, L., Eds.; Unesco: Paris, France, 1990; Volume 2, pp. 1061–1066. [Google Scholar]
- Szpilman, M. Peixes Marinhos do Brasil: Guia Prático de Identificação, 1st; Szpilman, M., Ed.; LTDA: São Paulo, Brazil, 2000; p. 288. [Google Scholar]
- Begossi, A.; Hanazaki, N.; Ramos, R.M. Healthy fish: Medicinal and recommended species in the amazon and the Atlantic Fores Coast (Brazil). In Eating and Healing: Traditional Food as Medicine, 1st; Pieroni, A., Price, L.L., Eds.; Haworth Press: Binghamton, NY, USA, 2006; pp. 237–250. [Google Scholar]
- Cavalli, L.S.; Possette, P.L.; Schmidt, B.; Kruel, C.; Grando, M.; Badiale Furlong, E.; Cezar-Vaz, M.R.; Barros, D.M.; Muccillo-Baisch, A.L. Fish Balistes capriscus skin extract-induced relaxation in mesenteric arterial bed of rat. J. Ethnopharmacol. 2003, 88, 215–220. [Google Scholar] [CrossRef]
- Muccilo-Baisch, A.L.; Silva, D.B.; Andrade, A.; Carrazzoni, D.; Vaz, M.R.C.; Furlong, E.B.; Soares, M.C.F. Effects of aqueous extract from Stephanolepis hispidus on blood pressure in the normal and in l-NAME-induced hypertensive rats. Online Braz. J. Nurs. 2007, 6. [Google Scholar] [CrossRef]
- Andrade, E.L.; Meotti, F.C.; Calixto, J.B. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2011, 133, 189–204. [Google Scholar] [CrossRef]
- Silva, J.P.; Rodarte, R.S.; Calheiros, A.S.; Souza, C.Z.; Amendoeira, F.C.; Martins, M.A.; Silva, P.M.; Frutuoso, V.S.; Barreto, E. Antinociceptive activity of aqueous extract of Bowdichia virgilioides in mice. J. Med. Food 2010, 13, 348–351. [Google Scholar] [CrossRef]
- Chau, T.T.; Weichman, B.M. Pemedolac: A novel and long-acting non-narcotic analgesic. J. Pharmacol. Exp. Ther. 1989, 248, 907–915. [Google Scholar]
- Neumann, P.B.; Henriksen, H.; Grosman, N.; Christensen, C.B. Plasma morphine concentrations during chronic oral administration in patients with cancer pain. Pain 1982, 13, 247–252. [Google Scholar] [CrossRef]
- Gibson, D.M.; Cotler, S.; Spiegel, H.E.; Colburn, W.A. Pharmacokinetics of recombinant leukocyte A interferon following various routes and modes of administration to the dog. J. Interferon Res. 1985, 5, 403–408. [Google Scholar] [CrossRef]
- Linley, J.E.; Rose, K.; Ooi, L.; Gamper, N. Understanding inflammatory pain: Ion channels contributing to acute and chronic nociception. Pflugers Arch. 2010, 459, 657–669. [Google Scholar] [CrossRef]
- Besson, J.M. The complexity of physiopharmacologic aspects of pain. Drugs 1997, 53, 1–9. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Kawabata, A. Prostaglandin E2 and pain—An update. Biol. Pharm. Bull. 2011, 34, 1170–1173. [Google Scholar] [CrossRef]
- Orlandi, L.; Vilela, F.C.; Santa-Cecilia, F.V.; Dias, D.F.; Alves-da-Silva, G.; Giusti-Paiva, A. Anti-Inflammatory and antinociceptive effects of the stem bark of byrsonima intermedia A. Juss. J. Ethnopharmacol. 2011, 137, 1469–1476. [Google Scholar] [CrossRef]
- Langerman, L.; Zakoeski, M.I.; Piskoun, B.; Grant, G.J. Hot plate versus tail flick: Evaluation of acute tolerance to continuous morphine infusion in the rat model. J. Pharmacol. Toxicol. Methods 1995, 34, 23–27. [Google Scholar] [CrossRef]
- Castro, L.S.; Perazzo, F.F.; Maistro, E.L. Genotoxicity testing of Ambelania occidentalis (Apocynaceae) leaf extract in vivo. Genet. Mol. Res. 2009, 8, 440–447. [Google Scholar] [CrossRef]
- Koster, R.M.; Anderson, M.; de Beer, E.J. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–418. [Google Scholar]
- Amendoeira, F.C.; Frutuoso, V.S.; Chedier, L.M.; Pearman, A.T.; Figueiredo, M.R.; Kaplan, M.A.; Prescott, S.M.; Bozza, P.T.; Castro-Faria-Neto, H.C. Antinociceptive effect of Nidularium procerum: A Bromeliaceae from the Brazilian coastal rain forest. Phytomedicine 2005, 12, 78–87. [Google Scholar] [CrossRef]
- Lavich, T.R.; Cordeiro, R.S.; Silva, P.M.; Martins, M.A. A novel hot-plate test sensitive to hyperalgesic stimuli and non-opioid analgesics. Braz. J. Med. Biol. Res. 2005, 38, 445–451. [Google Scholar]
- D’Amour, F.E.; Smith, D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941, 72, 74–79. [Google Scholar]
- Hartmann, A.; Speit, G. The contribution of cytotoxicity to DNA-effects in the single cell gel test (comet assay). Toxicol. Lett. 1997, 90, 183–188. [Google Scholar] [CrossRef]
- Speit, G.; Hartmann, A. The comet assay: A sensitive genotoxicity test for the detection of DNA damage and repair. Methods Mol. Biol. 2006, 314, 275–286. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sugiyama, C.; Morikawa, Y.; Hayashi, M.; Sofuni, T. A comparison between manual microscopic analysis and computerized image analysis in the single cell gel electrophoresis assay. MMS Commun. 1995, 3, 103–115. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carvalho, V.; Fernandes, L.; Conde, T.; Zamith, H.; Silva, R.; Surrage, A.; Frutuoso, V.; Castro-Faria-Neto, H.; Amendoeira, F. Antinociceptive Activity of Stephanolepis hispidus Skin Aqueous Extract Depends Partly on Opioid System Activation. Mar. Drugs 2013, 11, 1221-1234. https://doi.org/10.3390/md11041221
Carvalho V, Fernandes L, Conde T, Zamith H, Silva R, Surrage A, Frutuoso V, Castro-Faria-Neto H, Amendoeira F. Antinociceptive Activity of Stephanolepis hispidus Skin Aqueous Extract Depends Partly on Opioid System Activation. Marine Drugs. 2013; 11(4):1221-1234. https://doi.org/10.3390/md11041221
Chicago/Turabian StyleCarvalho, Vinicius, Lohengrin Fernandes, Taline Conde, Helena Zamith, Ronald Silva, Andrea Surrage, Valber Frutuoso, Hugo Castro-Faria-Neto, and Fabio Amendoeira. 2013. "Antinociceptive Activity of Stephanolepis hispidus Skin Aqueous Extract Depends Partly on Opioid System Activation" Marine Drugs 11, no. 4: 1221-1234. https://doi.org/10.3390/md11041221




