Structural Characteristics and Anticancer Activity of Fucoidan from the Brown Alga Sargassum mcclurei
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of Three Fucoidan Fractions from S. mcclurei
| Fucoidan | Eluent [NaCl], M | Yield, % * | Sugar total, % ** | SO3Na, % ** | Monosaccharide composition, mol% | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fuc | Man | Gal | Xyl | Glc | |||||
| SmF1 | 0.7–0.8 | 8.4 | 44.6 | 16.8 | 27.2 | 34.0 | 19.6 | 6.4 | 12.8 |
| SmF2 | 0.8–1.4 | 18.2 | 64.6 | 25.7 | 44.8 | 5.4 | 34.1 | 5.3 | 10.4 |
| SmF3 | 1.4–1.8 | 10.5 | 53.2 | 35.0 | 58.5 | 0 | 41.5 | 0 | 0 |
2.2. Structural Characteristics of the Fucoidan SmF3
| Partially methylated fucitol or galactitol acetates | mol% | Linkage type |
|---|---|---|
| 2,3,4-tri-O-methyl-fucitol | 5 | Fuc1→ |
| 2,3-di-O-methyl-fucitol | 12 | →4Fuc1→ |
| 2,4-di-O-methyl-fucitol | 46 | →3Fuc1→ |
| 2,3,4,6-tetra-O-methyl-galactitol | 8 | Gal1→ |
| 2,3,6-tri-O-methyl-galactitol | 18 | →4Gal1→ |
| 2,3,4-tri-O-methyl-galactitol | 2 | →6Gal1→ |
| 3,4-di-O-methyl-galactitol | 1 | →2,6Gal1→ |
| 2,4-di-O-methyl-galactitol | 2 | →3,6Gal1→ |
| 1,2,3,4-tetra-O-methyl-galactitol | 6 | →6Gal |
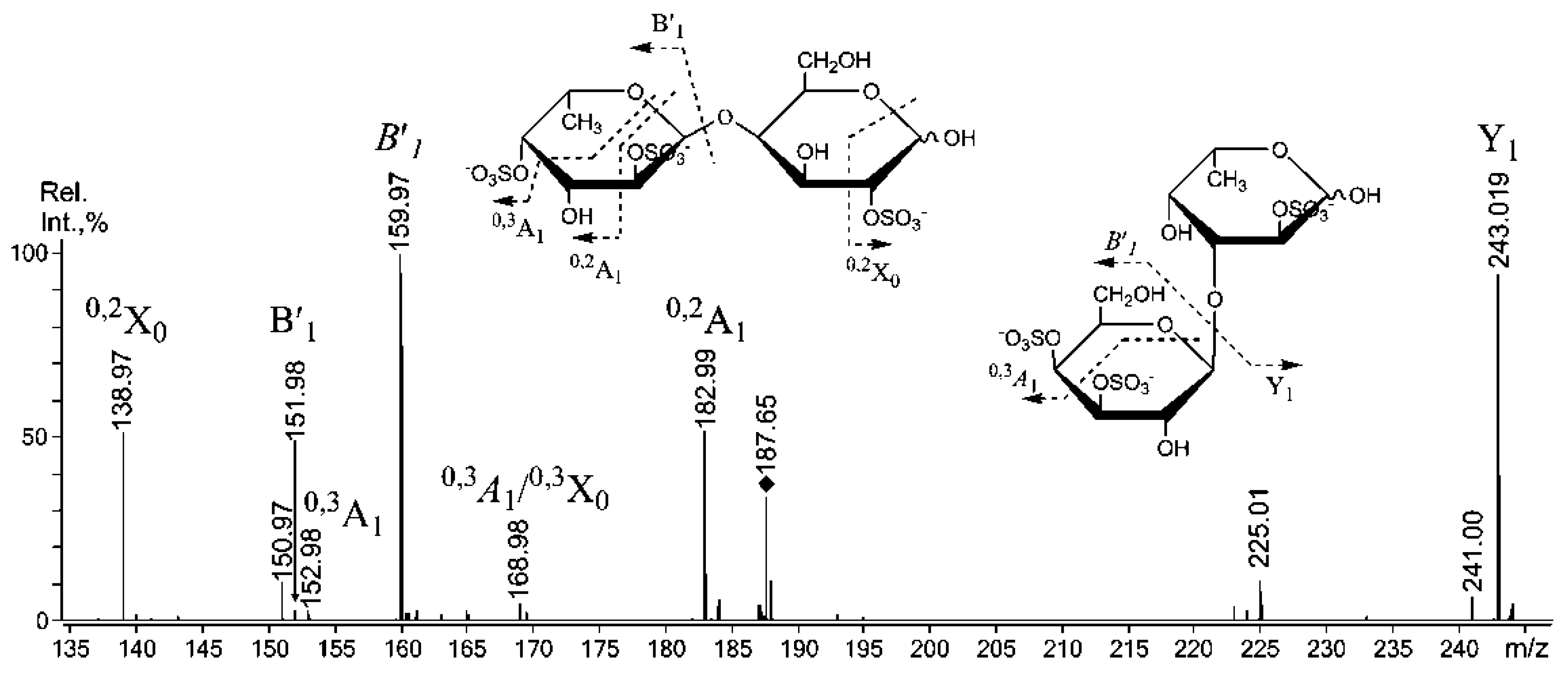
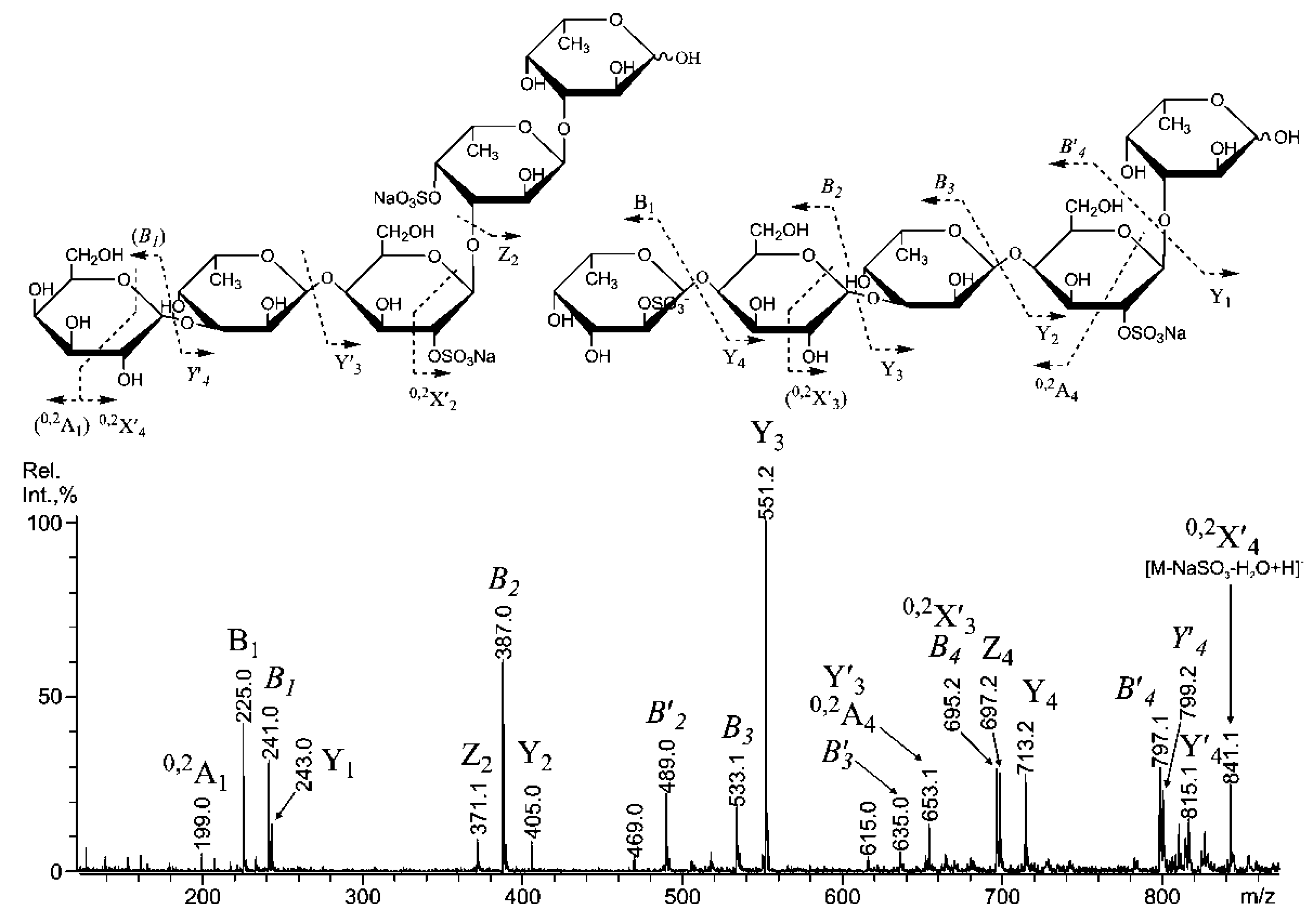
2.3. Mass Spectrometric Analysis of the Oligosaccharides Obtained by the Autohydrolysis of the Fucoidan SmF3
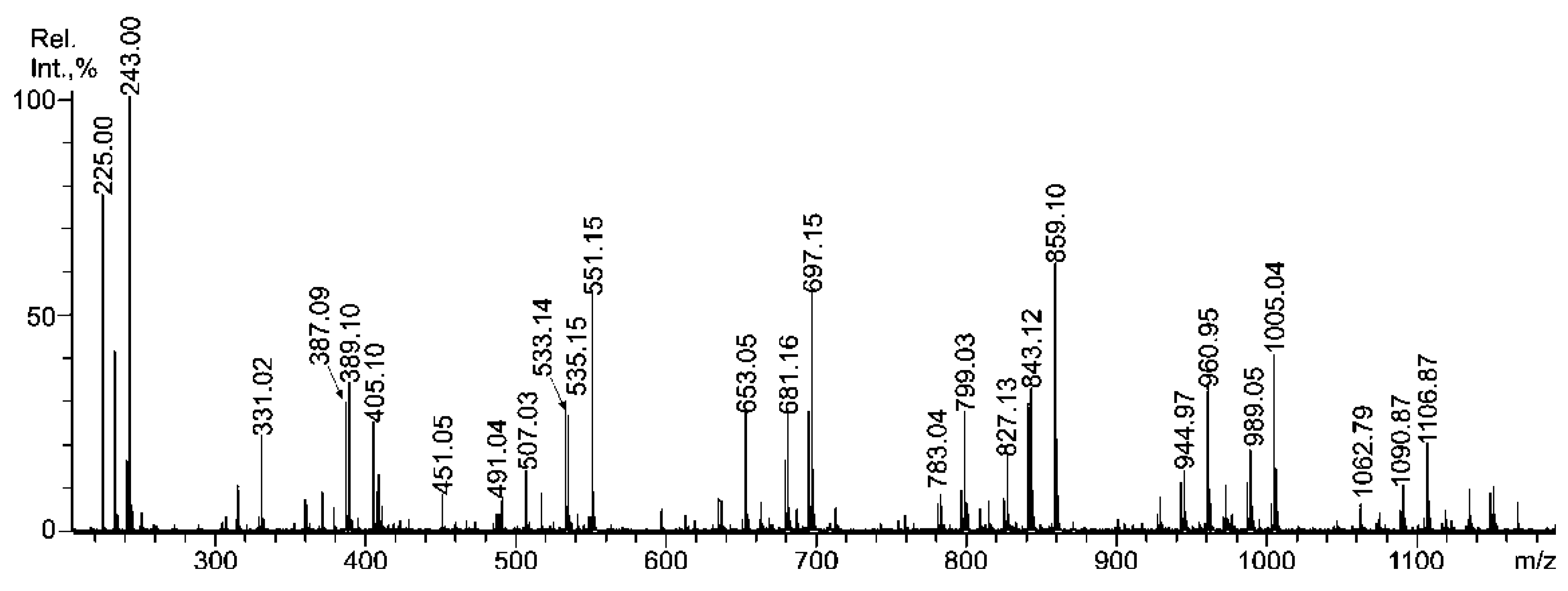
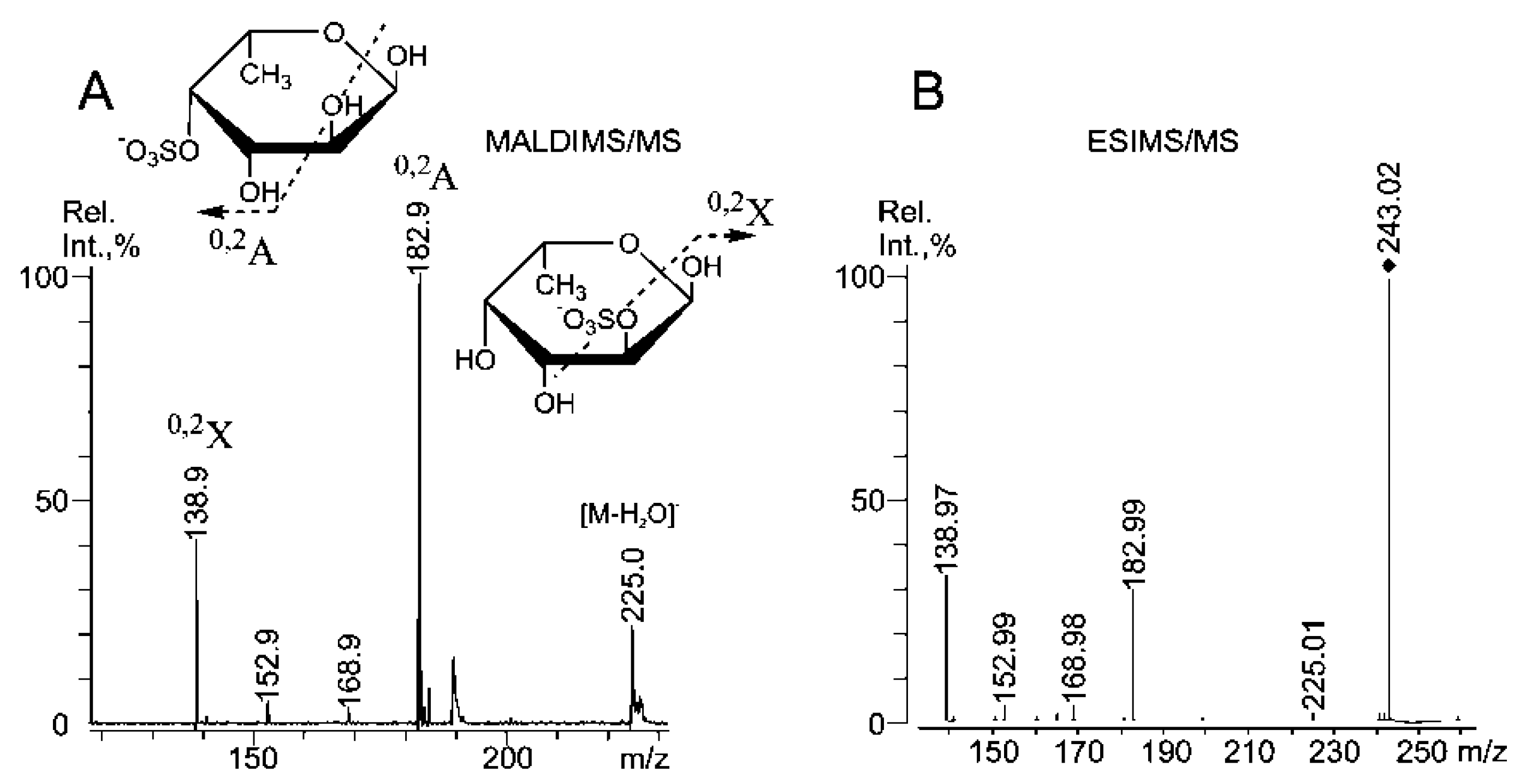
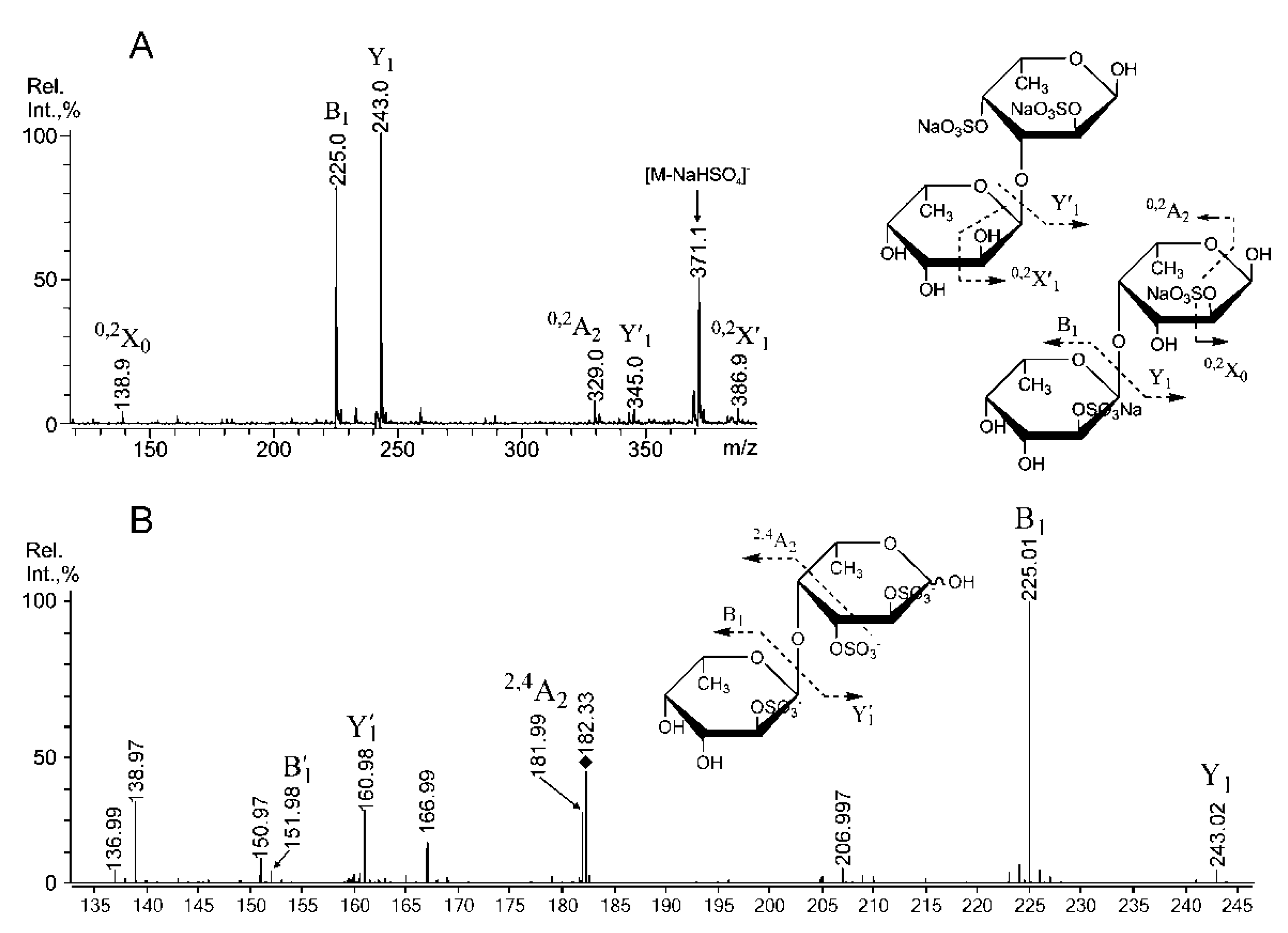
| m/z | Composition/structural features of mono- and oligosaccharides | |
|---|---|---|
| MALDI [M * − Na]− | ESI [M − nNa]n− | |
| 243.00 | 243.016 | Fuc(4SO3−), Fuc(2SO3−), Fuc(3SO3−) |
| - | 259.012 | Gal(4(6)SO3−), Gal(2SO3−) |
| 389.10 | 389.077 | [Fuc2(SO3)]− |
| 405.10 | 405.070 | Gal(2SO3−)-(1,3)-Fuc |
| 491.04 | 234.0102− | Fuc(2SO3−)-(1,3)-Fuc(2SO3−) Fuc-(1,3)-Fuc(2,4SO3−) Fuc(2SO3−)-(1,4)-Fuc(2SO3−) ** |
| - | 182.3233− | Fuc(2SO3−)-(1,4)-Fuc (2,3SO3−) Fuc(2,4SO3−)-(1,3)-Fuc ** |
| - | 187.6542− | Gal(4/6,3SO3−)-(1,3/4)-Fuc(2/3SO3−) Fuc(2,4SO3−)-(1,4)-Gal(2SO3−) |
| 507.03 | 242.008 | Gal(2SO3−)-(1,3)-Fuc(2SO3−) Gal(2SO3−)-(1,3)-Fuc(2SO3−) ** |
| 535.15 | 535.136 | [Fuc3(SO3)]− |
| - | 231.007 | [Fuc3(SO3)]3− |
| 551.15 | - | Gal(2SO3−)-(1,3)-Fuc-(1,3)-Fuc |
| - | 236.341 | [Fuc2Gal(SO3)3]3− |
| 653.05 | 315.0402− | [Fuc2Gal(SO3Na)2 − Na]− |
| 681.16 | - | [Fuc4(SO3)]− |
| 697.15 | - | [Fuc3Gal(SO3)]− |
| 783.04 | 380.0742− | [Fuc4(SO3Na)2 − Na]− |
| 799.03 | 388.0702− | [Fuc3Gal(SO3Na)2 − Na]− |
| - | 285.0283− | Gal(2SO3−)-(1,3)-Fuc-(1,3)-Fuc(SO3−)-(1,3)-Fuc(SO3−) Gal(4/6,2SO3−)-(1,3)-Fuc-(1,3)-Fuc-(1,3)-Fuc(2SO3−) Fuc-(1,4)-Gal(3SO3−)-(1,3)-Fuc(2SO3−)-(1,3)-Fuc(2SO3−) |
| 827.13 | - | [Fuc5(SO3)]− |
| 843.12 | - | [Fuc4Gal(SO3)]− |
| 859.10 | - | [Fuc3Gal2(SO3)]− |
| 944.97 | 460.0942− | [Fuc4Gal(SO3Na)2 − Na]− |
| 960.95 | - | [Fuc(2SO3Na)-(1,4)-Gal-(1,3)-Fuc(2SO3Na)-(1,4)-Gal-(1,3)-Fuc − Na]− [Fuc(2SO3Na)-(1,4)-Gal(2SO3Na)-(1,3)-Fuc-(1,4)-Gal-(1,3)-Fuc − Na]− [Gal(2SO3Na)-(1,3)-Fuc(2SO3Na)-(1,4)-Gal-(1,3)-Fuc-(1,3)-Fuc − Na]− [Gal(4SO3Na)-(1,3)-Fuc-(1,4)-Gal-(1,3)-Fuc(4SO3Na)-(1,3)-Fuc − Na]− |
| - | 339.0513− | [Fuc3Gal2(SO3)3]3− |
| 989.05 | - | [Fuc5Gal(SO3)− |
| 1005.04 | - | [Fuc4Gal2(SO3)]− |
| 1062.79 | 338.551 | [Fuc3Gal2(SO3Na)3 − Na]− |
| 1090.87 | - | [Fuc5Gal(SO3Na)2 − Na]− |
| 1106.87 | 541.065 | [Fuc4Gal2(SO3Na)2 − Na]− |

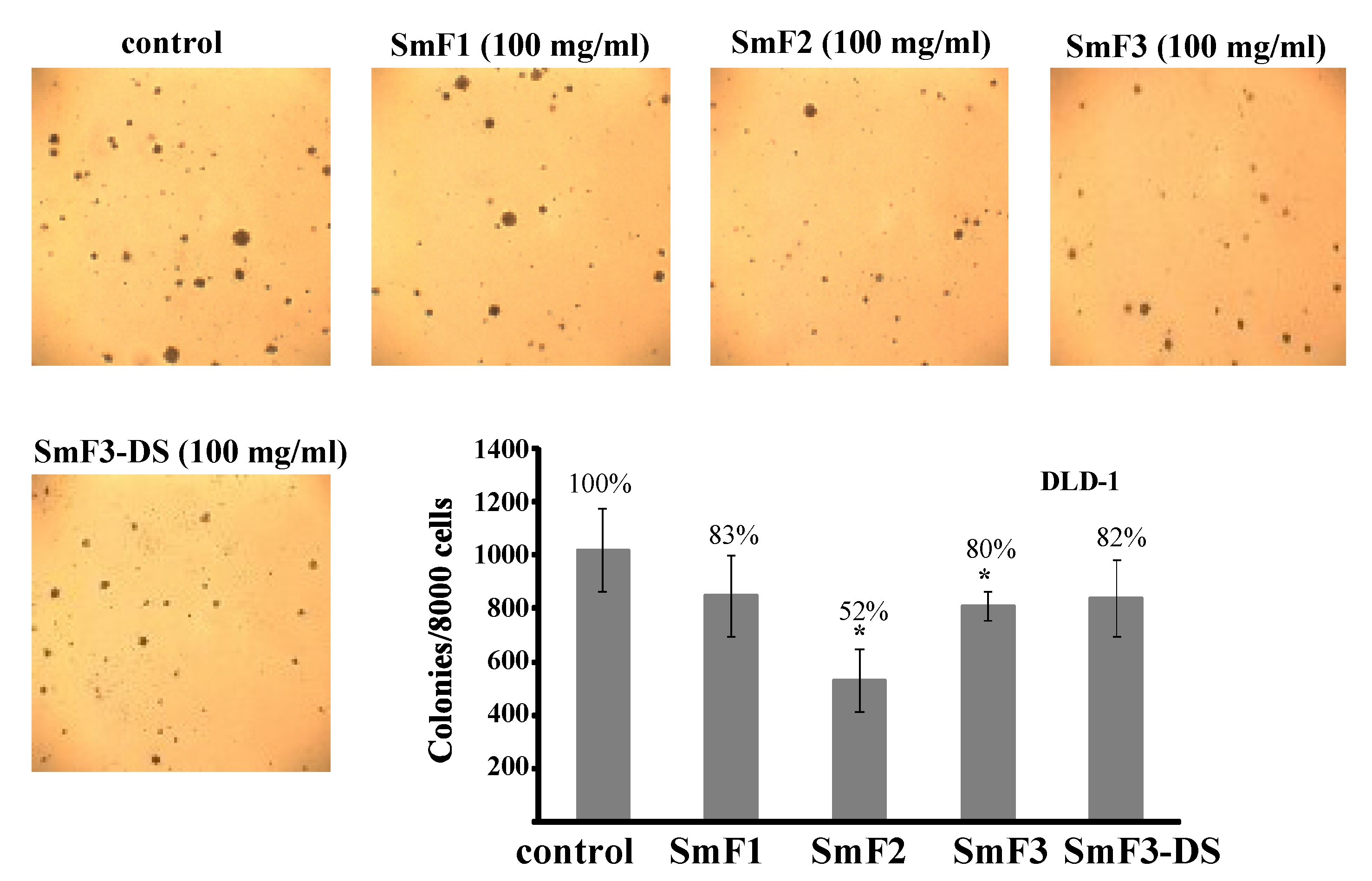
2.4. Cytotoxicity and Antitumor Activity of SmF1, SmF2, SmF3, and SmF3-DS
3. Experimental Section
3.1. Materials
3.2. Instruments
3.3. General Methods
3.3.1. Analytical Procedures
3.3.2. Mass Spectrometric Analysis
3.3.3. Water-Soluble Polysaccharide Extraction
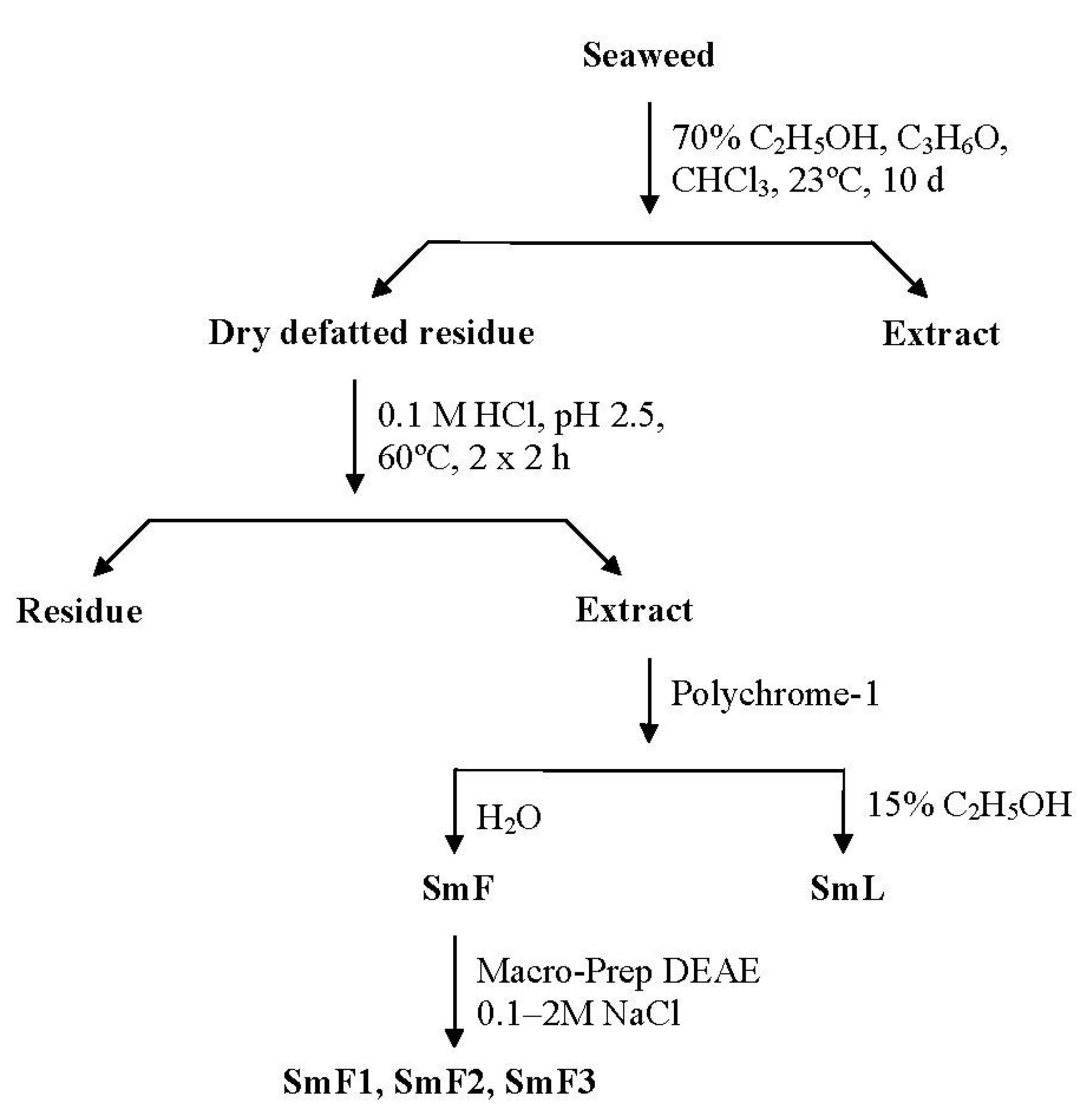
3.3.4. Hydrophobic Chromatography
3.3.5. Anion-Exchange Chromatography
3.3.6. Desulfation of Fucoidan
3.3.7. Methylation of Fucoidan
3.3.8. Depolymerization of Fucoidan by Autohydrolysis
3.4. Anticancer Activity
3.4.1. Cell Culture
3.4.2. Cell Cytotoxicity Assay
3.4.3. Soft Agar Clonogenic Assay
3.4.4. Data Analysis
4. Conclusions
Acknowledgements
References
- Anastyuk, S.D.; Shevchenko, N.M.; Ermakova, S.P.; Vishchuk, O.S.; Nazarenko, E.L.; Dmitrenok, P.S.; Zvyagintseva, T.N. Anticancer activity in vitro of a fucoidan from the brown alga Fucus evanescens and its low-molecular fragments, structurally characterized by tandem mass-spectrometry. Carbohydr. Polym. 2012, 87, 186–194. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Shevchenko, N.M.; Nazarenko, E.L.; Imbs, T.I.; Gorbach, V.I.; Dmitrenok, P.S.; Zvyagintseva, T.N. Structural analysis of a highly sulfated fucan from the brown alga Laminaria cichorioides by tandem MALDI and ESI mass spectrometry. Carbohydr. Res. 2010, 345, 2206–2212. [Google Scholar] [CrossRef]
- Kusaykin, M.; Bakunina, I.; Sova, V.; Ermakova, S.; Kuznetsova, T.; Besednova, N.; Zaporozhets, T.; Zvyagintseva, T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol. J. 2008, 3, 904–915. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.J.; Zhao, R.X. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.; Dantas-Santos, N.; Camara, R.B.; Cordeiro, S.L.; Costa, M.S.; Almeida-Lima, J.; Melo-Silveira, R.F.; Oliveira, R.M.; et al. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef]
- Mohsen, M.S.; Asker, S.F.; Mohamed, F.M.A.; Osama, H.E.S. Chemical structure and antiviral activity of water-soluble sulfated polysaccharides from Sargassum latifolium. J. Appl. Sci. Res. 2007, 3, 1178–1185. [Google Scholar]
- Preeprame, S.; Hayashi, K.; Lee, J.B.; Sankawa, U.; Hayashi, T. A novel antivirally active fucan sulfate derived from an edible brown alga, Sargassum horneri. Chem. Pharm. Bull. 2001, 49, 484–485. [Google Scholar] [CrossRef]
- Duarte, M.E.; Cardoso, M.A.; Noseda, M.D.; Cerezo, A.S. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Res. 2001, 333, 281–293. [Google Scholar] [CrossRef]
- Lee, J.B.; Takeshita, A.; Hayashi, K.; Hayashi, T. Structures and antiviral activities of polysaccharides from Sargassum trichophyllum. Carbohydr. Polym. 2011, 86, 995–999. [Google Scholar] [CrossRef]
- Ly, B.M.; Buu, N.Q.; Nhut, N.D.; Thinh, P.D.; Van, T.T.T. Studies on fucoidan and its production from Vietnamese brown seaweeds. ASEAN J. Sci. Technol. Dev. 2005, 22, 371–380. [Google Scholar]
- Sokolova, R.V.; Ermakova, S.P.; Awada, S.M.; Zvyagintseva, T.N. Composition, structural characteristics, and antitumor properties of polysaccharides from the brown algae Dictyopteris polypodioides and Sargassum sp. Chem. Nat. Comp. 2011, 47, 329–334. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Imbs, T.I.; Shevchenko, N.M.; Dmitrenok, P.S.; Zvyagintseva, T.N. ESIMS analysis of fucoidan preparations from Costaria costata, extracted from alga at different life-stages. Carbohydr. Polym. 2012, 90, 993–1002. [Google Scholar] [CrossRef]
- Imbs, T.I.; Shevchenko, N.M.; Sukhoverkhov, S.V.; Semenova, T.L.; Skriptsova, A.V.; Zvyagintseva, T.N. Seasonal variations of the composition and structural characteristics of polysaccharides from the brown alga Costaria costata. Chem. Nat. Comp. 2009, 45, 79–86. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Tarbeeva, D.V.; Zvyagintseva, T.N. Comparative study of polysaccharides from reproductive and sterile tissue of five brown seaweeds. Mar. Biotechnol. 2012, 14, 304–311. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M. Cancer statistics, 2008. Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96–107. [Google Scholar] [CrossRef]
- Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Boo, H.J.; Kwon, J.M.; Koh, Y.S.; Hyun, J.W.; Park, D.B.; Yoo, E.S.; et al. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1760–1764. [Google Scholar] [CrossRef]
- Nishino, T.; Nishioka, C.; Ura, H.; Nagumo, T. Isolation and partial characterization of a novel amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohydr. Res. 1994, 255, 213–224. [Google Scholar] [CrossRef]
- Vischuk, O.S.; Ermakova, S.P.; Tin, F.D.; Shevchenko, N.M.; Li, B.M.; Zvyagintseva, T.N. Anticancer activity of brown algae-derived fucoidans. Pac. Med. J. 2009, 3, 86–89. [Google Scholar]
- Ermakova, S.; Men’shova, R.; Vishchuk, O.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Water-soluble polysaccharides from the brown alga Eisenia bicyclis: Structural characteristics and antitumor activity. Algal Res. 2013, 2, 51–58. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The effect of sulfated (1→3)-α-l-fucan from the brown alga Saccharina cichorioides miyabe on resveratrol-induced apoptosis in colon carcinoma cells. Mar. Drugs 2013, 11, 194–212. [Google Scholar] [CrossRef]
- Cuicanu, I.; Kerek, F. A simple and rapid method for the permetylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Bjorndal, H.; Hellerquist, C.G.; Lindberg, B.; Svensson, S. Gas-liquid chromatography and mass spectrometry in methylation analysis of polysaccharides. Angew. Chem. Int. Ed. Engl. 1970, 9, 610–619. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, H.; Niu, X. Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica. Int. J. Biol. Macromol. 2010, 47, 126–131. [Google Scholar] [CrossRef]
- Shevchenko, N.M.; Anastyuk, S.D.; Gerasimenko, N.I.; Dmitrenok, P.S.; Isakov, V.V.; Zvyagintseva, T.N. Polysaccharide and lipid composition of the brown seaweed Laminaria gurjanovae. Russ. J. Bioorgan. Chem. 2007, 33, 88–98. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Shevchenko, N.M.; Dmitrenok, P.S.; Zvyagintseva, T.N. Structural similarities of fucoidans from brown algae Silvetia babingtonii and Fucus evanescens, determined by tandem MALDI-TOF mass spectrometry. Carbohydr. Res. 2012, 358, 78–81. [Google Scholar] [CrossRef]
- Zaia, J. Mass spectrometry of oligosaccharides. Mass Spectrom. Rev. 2004, 23, 161–227. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in Fab-Ms Ms spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, X.; Yang, B.; Ren, S.; Guan, H.; Zhang, Y.; Lawson, A.M.; Chai, W. Sequence determination of sulfated carrageenan-derived oligosaccharides by high-sensitivity negative-ion electrospray tandem mass spectrometry. Anal. Chem. 2006, 78, 8499–8505. [Google Scholar] [CrossRef]
- Saad, O.M.; Leary, J.A. Delineating mechanisms of dissociation for isomeric heparin disaccharides using isotope labeling and ion trap tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 1274–1286. [Google Scholar] [CrossRef]
- Tissot, B.; Salpin, J.Y.; Martinez, M.; Gaigeot, M.P.; Daniel, R. Differentiation of the fucoidan sulfated l-fucose isomers constituents by CE-ESIMS and molecular modeling. Carbohydr. Res. 2006, 341, 598–609. [Google Scholar] [CrossRef]
- Minamisawa, T.; Hirabayashi, J. Fragmentations of isomeric sulfated monosaccharides using electrospray ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1788–1796. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Shevchenko, N.M.; Nazarenko, E.L.; Dmitrenok, P.S.; Zvyagintseva, T.N. Structural analysis of a fucoidan from the brown alga Fucus evanescens by MALDI-TOF and tandem ESI mass spectrometry. Carbohydr. Res. 2009, 344, 779–787. [Google Scholar] [CrossRef]
- Daniel, R.; Chevolot, L.; Carrascal, M.; Tissot, B.; Mourao, P.A.S.; Abian, J. Electrospray ionization mass spectrometry of oligosaccharides derived from fucoidan of Ascophyllum nodosum. Carbohydr. Res. 2007, 342, 826–834. [Google Scholar] [CrossRef]
- Yang, C.; Chung, D.; Shin, I.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ermakova, S.P.; Zvyagintseva, T.N.; Kang, K.W.; Dong, Z.; Choi, H.S. Inhibitory effects of fucoidan on activation of epidermal growth factor receptor and cell transformation in JB6 Cl41 cells. Food Chem. Toxicol. 2008, 46, 1793–1800. [Google Scholar] [CrossRef]
- Fukahori, S.; Yano, H.; Akiba, J.; Ogasawara, S.; Momosaki, S.; Sanada, S.; Kuratomi, K.; Ishizaki, Y.; Moriya, F.; Yagi, M.; et al. Fucoidan, a major component of brown seaweeds, prohibits the growth of human cancer cells in vitro. Mol. Med. Rep. 2008, 1, 537–542. [Google Scholar]
- Chen, P.; Baker, A.G.; Novotny, M.V. The use of osazones as matrices for the matrix-assisted laser desorption/ionization mass spectrometry of carbohydrates. Anal. Biochem. 1997, 244, 144–151. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, T.V. Marine Plants of the Asian-Pacific Region Countries, Their Use and Cultivation; Dalnauka: Vladivostok, Russia, 2012; p. 377. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of totalphenolics with phospho-molybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar]
- Anastyuk, S.D.; Barabanova, A.O.; Correc, G.; Nazarenko, E.L.; Davydova, V.N.; Helbert, W.; Dmitrenok, P.S.; Yermak, I.M. Analysis of structural heterogeneity of κ/β-carrageenan oligosaccharides from Tichocarpus crinitus by negative-ion ESI and tandem MALDI mass spectrometry. Carbohydr. Polym. 2011, 86, 546–554. [Google Scholar] [CrossRef]
- Colburn, N.H.; Wendel, E.J.; Abruzzo, G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: Mitogen-resistant variants are sensitive to promotion. Proc. Natl. Acad. Sci. USA 1981, 78, 6912–6916. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural Characteristics and Anticancer Activity of Fucoidan from the Brown Alga Sargassum mcclurei. Mar. Drugs 2013, 11, 1456-1476. https://doi.org/10.3390/md11051456
Thinh PD, Menshova RV, Ermakova SP, Anastyuk SD, Ly BM, Zvyagintseva TN. Structural Characteristics and Anticancer Activity of Fucoidan from the Brown Alga Sargassum mcclurei. Marine Drugs. 2013; 11(5):1456-1476. https://doi.org/10.3390/md11051456
Chicago/Turabian StyleThinh, Pham Duc, Roza V. Menshova, Svetlana P. Ermakova, Stanislav D. Anastyuk, Bui Minh Ly, and Tatiana N. Zvyagintseva. 2013. "Structural Characteristics and Anticancer Activity of Fucoidan from the Brown Alga Sargassum mcclurei" Marine Drugs 11, no. 5: 1456-1476. https://doi.org/10.3390/md11051456




