Alternative Sources of n-3 Long-Chain Polyunsaturated Fatty Acids in Marine Microalgae
Abstract
:1. Introduction
2. Long Chain Polyunsaturated Fatty Acids and Their Importance in Human Health
3. LC n-3 PUFA Sources: The Need for Alternatives
4. Microalgae Production
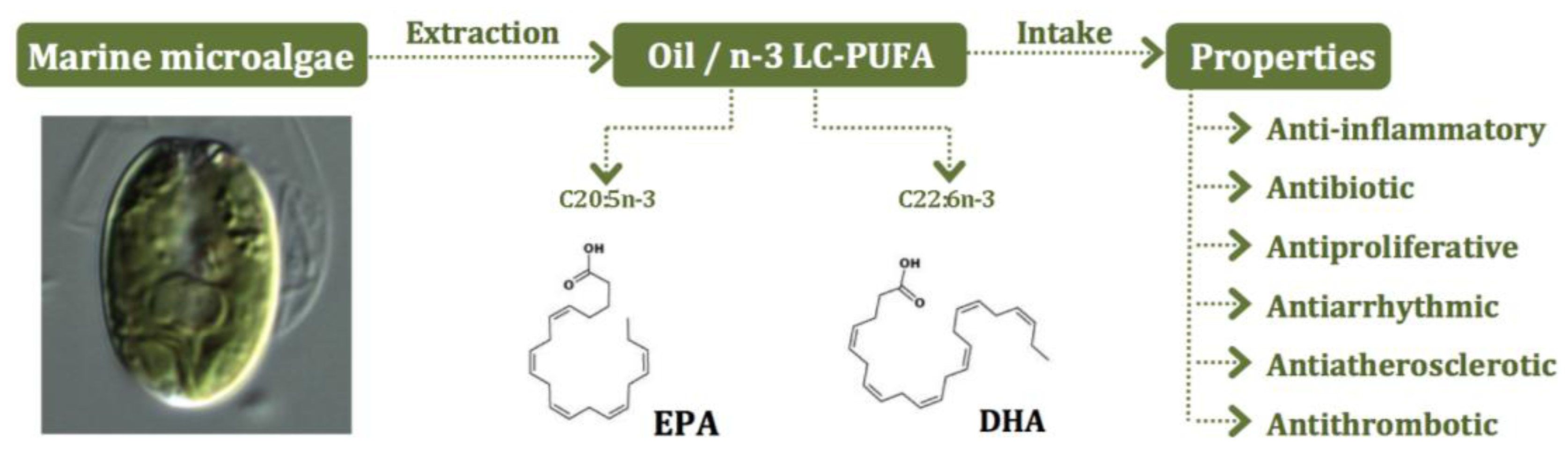
5. Microalgae as Sources of n-3 LC-PUFA
| Company and commercial product designation | % EPA or DHA 2 | Microbial sources available | Comments |
|---|---|---|---|
| Aurora Algae A2 EPA Pure™ | 65% EPA (regular) 95% EPA (pharma) | Undisclosed | Phototrophic, open-pond |
| Qualitas Health EicoOil™ | 25%–30% EPA | Nannochloropsis oculata Hibberd | Phototrophic, open-pond |
| Algae Biosciences AlgaeBio Omega-3 Origins™ | 20% EPA; 20% DHA | Undisclosed | Oil blend from two marine strains |
| DSM-NP life’s DHA™ | 40%–45% DHA | Crypthecodinium cohnii Javornicky | Heterotrophic fermentation |
| DSM-NP life’s DHA plus EPA™ | 10% EPA; 22.5% DHA | Schizochytrium sp. Goldstein and Belsky | |
| Lonza DHAid™ | 35%–40% DHA | Ulkenia sp. Gaertner | Heterotrophic fermentation |
| Source-Omega Source Oil™ | 35%–40% DHA | Schizochytrium sp. Goldstein and Belsky | Heterotrophic fermentation |
| GCI Nutrients DHA Algae 35% Oil | 35% DHA | Crypthecodinium cohnii Javornicky | Heterotrophic fermentation |

5.1. Photoautotrophic Microalgae
| Species | EPA content (% TFA) 1 | EPA content (% DW) 2 | References |
|---|---|---|---|
| Nannochloropsis sp. Hibberd | 38–39 | 2–3 | [72] |
| 15–18 | 5–6 | [73] | |
| 11–22 | 3–6 | [74] | |
| 15–27 | 4 | [75] | |
| 5–27 | 2–4 | [69] | |
| 30–35 | 3–4 | Soley Biotechnology Institute 3 | |
| 35–39 | 4 | Soley Biotechnology Institute 3 | |
| Phaeodactylum tricornutum Bohlin | 31 | 5 | [76] |
| 40–57 | 1–4 | [77] | |
| 28 | 3 | [78] | |
| 30–32 | 3 | Soley Biotechnology Institute 3 | |
| 38–42 | 4–5 | Soley Biotechnology Institute 3 | |
| Nitzschia laevis Hustedt | 25–33 | 3–4 | [79] |
| 11–16 | 2–3 | [80] | |
| Porphyridium cruentum Nägeli | 25 | 3 | [81] |
| 41 | - | [82] | |
| Odontella aurita Agardh | 26 | - | [83] |
| Pavlova lutheri Green | 18–23 | - | [83] |
| 22–29 | - | [84] | |
| Cyclotella cryptica Lewin and Guillard | 17–23 | 1 | [85] |
| Cylindrotheca sp. Rabenhorst | 24–25 | - | [86] |
5.2. Heterotrophic Microalgae
| Species | DHA content (% TFA) 1 | DHA content (% DW) 2 | References |
|---|---|---|---|
| Schizochytrium mangrovei Raghuk | 31–41 | 12–21 | [100] |
| Schizochytrium limacinum Honda and Yokochi | 25–35 | 5–15 | [101] |
| - | 15–19 | [102] | |
| Schizochytrium sp. (HX-308) Goldstein and Belsky | 40–56 | 11–20 | [103] |
| Schizochytrium sp. Goldstein and Belsky | 45–52 | 20–24 | [104] |
| 28 | 4 | [100] | |
| Thraustochytrium sp. Sparrow | 23–24 | 16–17 | [105] |
| Thraustochytrium aureum Goldstein | 32–37 | 6–7 | [106] |
| Thraustochytrium striatum Schneider | 37 | 2 | [100] |
| Ulkenia sp. Gaertner | 10–23 | 5 | [107] |
| Aurantiochytrium sp. Yokoyama and Honda | 40 | 18 | [108] |
| Crypthecodinium cohnii Javornicky | 19–34 | 2–4 | [109] |
| 63 | 6 | [110] | |
| 53–57 | 5–6 | [111] |
5.3. Extraction and Quantification of PUFA in Microalgae
6. Future Perspectives and Conclusion
Acknowledgments
Conflict of Interest
References
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications. Renew. Sust. Energ. Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Kainz, M.J.; Fisk, A.T. Integrating Lipids and Contaminants in Aquatic Ecology and Ecotoxicology. In Lipids in Aquatic Ecosystems; Arts, M.T., Brett, M.T., Kainz, M., Eds.; Springer: New York, NY, USA, 2009; pp. 93–114. [Google Scholar]
- Sasso, S.; Pohnert, G.; Lohr, M.; Mittag, M.; Hertweck, C. Microalgae in the postgenomic era: A blooming reservoir for new natural products. FEMS Microbiol. Rev. 2012, 36, 761–785. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Iskandarov, U.; Cohen, Z. LC-PUFA from photosynthetic microalgae: Occurrence, biosynthesis, and prospects in biotechnology. Appl. Microbiol. Biot. 2011, 91, 905–915. [Google Scholar] [CrossRef]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 1–10. [Google Scholar] [CrossRef]
- Schmidt, E.B.; Christensen, J.H.; Aardestrup, I.; Madsen, T.; Riahi, S.; Hansen, V.E.; Skou, H.A. Marine n-3 fatty acids: Basic features and background. Lipids 2001, 36, S65–S68. [Google Scholar] [CrossRef]
- Wilber, C.G.; Levine, V.E. Fat metabolism in Alaskan eskimos. Exp. Med. Surg. 1950, 8, 422–425. [Google Scholar]
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Moncada, S.; Vane, J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis. Lancet 1978, 2, 117–119. [Google Scholar]
- Larsen, R.; Eilertsen, K.-E.; Elvevoll, E.O. Health benefits of marine foods and ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef]
- Leaf, A.; Weber, P.C. A new era for science in nutrition. Am. J. Clin. Nutr. 1987, 45, 1048–1053. [Google Scholar]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Lands, W.E.M. Human Life: Caught in the Food Web. In Lipids in Aquatic Ecosystems; Arts, M.T., Brett, M.T., Kainz, M.J., Eds.; Springer: New York, NY, USA, 2009; pp. 327–354. [Google Scholar]
- Bernstein, A.M.; Sun, Q.; Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Willett, W.C. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010, 122, 876–883. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Nieminen, L.R.G.; Blasbalg, T.L.; Riggs, J.A.; Lands, W.E.M. Healthy intakes of n-3 and n-6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006, 83, 1483–1493. [Google Scholar]
- Pottala, J.V.; Garg, S.; Cohen, B.E.; Whooley, M.A.; Harris, W.S. Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease the heart and soul study. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 406–412. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A. Diet and lifestyle recommendations revision 2006—A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Jacobson, T.A. Secondary prevention of coronary artery disease with omega-3 fatty acids. Am. J. Cardiol. 2006, 98, 61–70. [Google Scholar] [CrossRef]
- Robinson, J.G.; Stone, N.J. Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. Am. J. Cardiol. 2006, 98, 39–49. [Google Scholar] [CrossRef]
- Patel, J.V.; Tracey, I.; Hughes, E.A.; Lip, G.Y. Omega-3 polyunsaturated acids and cardiovascular disease: Notable ethnic differences or unfulfilled promise? J. Thromb. Haemost. 2010, 8, 2095–2104. [Google Scholar] [CrossRef]
- Connor, W.E.; Connor, S.L. The importance of fish and docosahexaenoic acid in Alzheimer disease. Am. J. Clin. Nutr. 2007, 85, 929–930. [Google Scholar]
- Prior, P.L.; Galduroz, J.C.F. (N-3) Fatty acids: Molecular role and clinical uses in psychiatric disorders. Adv. Nutr. 2012, 3, 257–265. [Google Scholar] [CrossRef]
- Serini, S.; Fasano, E.; Piccioni, E.; Cittadini, A.R.M.; Calviello, G. Differential anti-cancer effects of purified EPA and DHA and possible mechanisms involved. Curr. Med. Chem. 2011, 18, 4065–4075. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Harvey, K.A.; Xu, Z.D.; Bammerlin, E.M.; Walker, C.; Altenburg, J.D. Docosahexaenoic acid: A natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. BioFactors 2011, 37, 399–412. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Campoy, C.; Escolano-Margarit, M.V.; Anjos, T.; Szajewska, H.; Uauy, R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br. J. Nutr. 2012, 107, S85–S106. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef]
- FDA, Food and Drug Administration, Notice of a Claim of GRAS Status; Food and Drug Administration: Silver Spring, MD, USA, 1998.
- Jacobsen, C. Enrichment of Foods with Omega-3 Fatty Acids: A Multidisciplinary Challenge. In Foods for Health in the 21st Century: A Road Map for the Future; Gershwin, M.E., Greenwood, M.R.C., Eds.; Wiley Blackwell: New York, NY, USA, 2010. [Google Scholar]
- Gerber, L.R.; Karimi, R.; Fitzgerald, T.P. Sustaining seafood for public health. Front. Ecol. Environ. 2012, 10, 487–493. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health—Evaluating the risks and the benefits. J. Am. Med. Assoc. 2006, 296, 1885–1899. [Google Scholar] [CrossRef]
- Ginsberg, G.L.; Toal, B.F. Quantitative approach for incorporating methylmercury risks and omega-3 fatty acid benefits in developing species-specific fish consumption advice. Environ. Health Persp. 2009, 117, 267–275. [Google Scholar]
- Tur, J.A.; Bibiloni, M.M.; Sureda, A.; Pons, A. Dietary sources of omega 3 fatty acids: Public health risks and benefits. Br. J. Nutr. 2012, 107, S23–S52. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations, The State of World Fisheries and Aquaculture 2012; FAO: Rome, Italy, 2012.
- Sayanova, O.; Napier, J.A. Transgenic oilseed crops as an alternative to fish oils. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 253–260. [Google Scholar] [CrossRef]
- Meesapyodsuk, D.; Qiu, X. The front-end desaturase: Structure, function, evolution and biotechnological use. Lipids 2012, 47, 227–237. [Google Scholar] [CrossRef]
- Wu, X.; Ouyang, H.; Duan, B.; Pang, D.; Zhang, L.; Yuan, T.; Xue, L.; Ni, D.; Cheng, L.; Dong, S.; et al. Production of cloned transgenic cow expressing omega-3 fatty acids. Transgenic Res. 2012, 21, 537–543. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Dou, H.; Yin, J.; Chen, Y.; Pang, X.; Vajta, G.; Bolund, L.; Du, Y.; Ma, R.Z. Handmade cloned transgenic piglets expressing the nematode fat-1 gene. Cell. Reprogram. 2012, 14, 258–266. [Google Scholar]
- Sloan, E. Top 10 functional food trends. Food Technol. 2012, 66, 33–62. [Google Scholar]
- Apt, K.E.; Behrens, P.W. Commercial developments in microalgal biotechnology. J. Phycol. 1999, 35, 215–226. [Google Scholar] [CrossRef]
- Lee, Y.K. Commercial production of microalgae in the Asia-Pacific rim. J. Appl. Phycol. 1997, 9, 403–411. [Google Scholar] [CrossRef]
- Bumbak, F.; Cook, S.; Zachleder, V.; Hauser, S.; Kovar, K. Best practices in heterotrophic high-cell-density microalgal processes: Achievements, potential and possible limitations. Appl. Microbiol. Biot. 2011, 91, 31–46. [Google Scholar] [CrossRef]
- Zhou, P.-P.; Lu, M.-B.; Li, W.; Yu, L.-J. Microbial production of docosahexaenoic acid by a low temperature-adaptive strain Thraustochytriidae sp. Z105: Screening and optimization. J. Basic Microbiol. 2010, 50, 380–387. [Google Scholar]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar]
- Shene, C.; Leyton, A.; Esparza, Y.; Flores, L.; Quilodran, B.; Hinzpeter, I.; Rubilar, M. Microbial oils and fatty acids: Effect of carbon source on docosahexaenoic acid (C22:6 n-3, DHA) production by thraustochytrid strains. J. Soil Sci. Plant Nutr. 2010, 10, 207–216. [Google Scholar]
- Brett, M.T.; Muller-Navarra, D.C. The role of highly unsaturated fatty acids in aquatic food web processes. Freshw. Biol. 1997, 38, 483–499. [Google Scholar] [CrossRef]
- Thompson, G.A. Lipids and membrane function in green algae. Lipids Lipid Metab. 1996, 1302, 17–45. [Google Scholar] [CrossRef]
- Bigogno, C.; Khozin-Goldberg, I.; Boussiba, S.; Vonshak, A.; Cohen, Z. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 2002, 60, 497–503. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 1–16. [Google Scholar] [CrossRef]
- Grebow, J. Omegas everywhere. Nutr. Outlook Online 2012, 15, 24–40. [Google Scholar]
- Metz, J.G.; Roessler, P.; Facciotti, D.; Levering, C.; Dittrich, F.; Lassner, M.; Valentine, R.; Lardizabal, K.; Domergue, F.; Yamada, A.; et al. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 2001, 293, 290–293. [Google Scholar] [CrossRef]
- Sayanova, O.; Haslam, R.P.; Caleron, M.V.; Ruiz-Lopez, N.; Worthy, C.; Rooks, P.; Allen, M.J.; Napier, J.A. Identification and functional characterisation of genes encoding the omega-3 polyunsaturated fatty acid biosynthetic pathway from the coccolithophore Emiliania huxleyi. Phytochemistry 2011, 72, 594–600. [Google Scholar] [CrossRef]
- Pond, D.W.; Harris, R.P. The lipid composition of the coccolithophore Emiliania huxleyi and its possible ecophysiological significance. J. Mar. Biol. Assoc. UK 1996, 76, 579–594. [Google Scholar] [CrossRef]
- Luthria, D.L.; Mohammed, B.S.; Sprecher, H. Regulation of the biosynthesis of 4,7,10,13,16,19-docosahexaenoic acid. J. Biol. Chem. 1996, 271, 16020–16025. [Google Scholar]
- Chi, X.; Zhang, X.; Guan, X.; Ding, L.; Li, Y.; Wang, M.; Lin, H.; Qin, S. Fatty acid biosynthesis in eukaryotic photosynthetic microalgae: Identification of a microsomal delta 12 desaturase in Chlamydomonas reinhardtii. J. Microbiol. 2008, 46, 189–201. [Google Scholar] [CrossRef]
- Wynn, J.P.; Ratledge, C. Oils from Microorganisms. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Cohen, Z. Production of Polyunsaturated Fatty Acids by the Microalga Porphyridium cruentum. In Production of Chemicals by Microalgae; Cohen, Z., Ed.; Taylor and Francis: London, UK, 1999. [Google Scholar]
- Iida, I.; Nakahara, T.; Yokochi, T.; Kamisaka, Y.; Yagi, H.; Yamaoka, M.; Suzuki, M. Improvement of docosahexaenoic acid production in a culture of Thraustochytrium aureum by medium optimization. J. Ferment. Bioeng. 1996, 81, 76–78. [Google Scholar] [CrossRef]
- Cohen, Z.; Khozin-Goldberg, I.; Adlerstein, D.; Bigogno, C. The role of triacylglycerol as a reservoir of polyunsaturated fatty acids for the rapid production of chloroplastic lipids in certain microalgae. Biochem. Soc. Trans. 2000, 28, 740–743. [Google Scholar] [CrossRef]
- Tonon, T.; Harvey, D.; Larson, T.R.; Graham, I.A. Long chain polyunsaturated fatty acid production and partitioning to triacylglycerols in four microalgae. Phytochemistry 2002, 61, 15–24. [Google Scholar]
- Gog, A.; Senila, L.; Roman, M.; Luca, E.; Roman, C.; Irimie, F.-D. Oil extraction and fatty acid characterization of Nannochloropsis oculata microalgae for biodiesel applications. Stud. Univ. Babes-Bol. 2012, 57, 111–118. [Google Scholar]
- Gu, N.; Lin, Q.; Li, G.; Tan, Y.; Huang, L.; Lin, J. Effect of salinity on growth, biochemical composition, and lipid productivity of Nannochloropsis oculata CS 179. Eng. Life Sci. 2012, 12, 631–637. [Google Scholar] [CrossRef]
- Hu, H.; Gao, K. Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol. Lett. 2006, 28, 987–992. [Google Scholar] [CrossRef]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biot. 2011, 90, 1429–1441. [Google Scholar] [CrossRef]
- Sukenik, A.; Carmeli, Y. Lipid synthesis and fatty acid composition in Nannochloropsis sp. (Eustigmatophyceae) grown in a light-dark cycle. J. Phycol. 1990, 26, 463–469. [Google Scholar]
- Richmond, A.; Zhang, C.W.; Zarmi, Y. Efficient use of strong light for high photosynthetic productivity: Interrelationships between the optical path, the optimal population density and cell-growth inhibition. Biomol. Eng. 2003, 20, 229–236. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Fujita, Y. Isolation of enhanced eicosapentaenoic acid producing mutants of Nannochloropsis oculata ST-6 using ethyl methane sulfonate induced mutagenesis techniques and their characterization at mRNA transcript level. Phycol. Res. 2006, 54, 208–219. [Google Scholar] [CrossRef]
- Xu, F.; Hu, H.H.; Cong, W.; Cai, Z.L.; Ouyang, F. Growth characteristics and eicosapentaenoic acid production by Nannochloropsis sp in mixotrophic conditions. Biotechnol. Lett. 2004, 26, 51–53. [Google Scholar]
- Fang, X.; Wei, C.; Cai, Z.L.; Fan, O. Effects of organic carbon sources on cell growth and eicosapentaenoic acid content of Nannochloropsis sp. J. Appl. Phycol. 2004, 16, 499–503. [Google Scholar] [CrossRef]
- Zittelli, G.C.; Lavista, F.; Bastianini, A.; Rodolfi, L.; Vincenzini, M.; Tredici, M.R. Production of eicosapentaenoic acid by Nannochloropsis sp cultures in outdoor tubular photobioreactors. J. Biotechnol. 1999, 70, 299–312. [Google Scholar] [CrossRef]
- Meiser, A.; Schmid-Staiger, U.; Trosch, W. Optimization of eicosapentaenoic acid production by Phaeodactylum tricornutum in the flat panel airlift (FPA) reactor. J. Appl. Phycol. 2004, 16, 215–225. [Google Scholar] [CrossRef]
- Fernandez, F.G.A.; Perez, J.A.S.; Sevilla, J.M.F.; Camacho, F.G.; Grima, E.M. Modeling of eicosapentaenoic acid (EPA) production from Phaeodactylum tricornutum cultures in tubular photobioreactors. Effects of dilution rate, tube diameter, and solar irradiance. Biotechnol. Bioeng. 2000, 68, 173–183. [Google Scholar]
- Yongmanitchai, W.; Ward, O.P. Growth of and omega-3-fatty-acid production by Phaeodactylum tricornutum under different culture conditions. Appl. Environ. Microb. 1991, 57, 419–425. [Google Scholar]
- Cao, X.-H.; Li, S.-Y.; Wang, C.-L.; Lu, M.-F. Potential use of the herbicide quizalofop-p-ethyl for eicosapentaenoic acid overproduction by the diatom Nitzschia laevis. Chin. J. Biotechnol. 2007, 23, 885–890. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Jiang, Y.; Chen, F. High cell density culture of the diatom Nitzschia laevis for eicosapentaenoic acid production: Fed-batch development. Process Biochem. 2002, 37, 1447–1453. [Google Scholar] [CrossRef]
- Durmaz, Y.; Monteiro, M.; Bandarra, N.; Gokpinar, S.; Isik, O. The effect of low temperature on fatty acid composition and tocopherols of the red microalga, Porphyridium cruentum. J. Appl. Phycol. 2007, 19, 223–227. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Yu, H.Z.; Adlerstein, D.; Didi-Cohen, S.; Heimer, Y.M.; Cohen, Z. Triacylglycerols of the red microalga Porphyridium cruentum can contribute to the biosynthesis of eukaryotic galactolipids. Lipids 2000, 35, 881–889. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Fouqueray, M.; Mimouni, V.; Ulmann, L.; Jacquette, B.; Tremblin, G. Effect of UV stress on the fatty acid and lipid class composition in two marine microalgae Pavlova lutheri (Pavlovophyceae) and Odontella aurita (Bacillariophyceae). J. Appl. Phycol. 2010, 22, 629–638. [Google Scholar] [CrossRef]
- Guiheneuf, F.; Mimouni, V.; Ulmann, L.; Tremblin, G. Combined effects of irradiance level and carbon source on fatty acid and lipid class composition in the microalga Pavlova lutheri commonly used in mariculture. J. Exp. Mar. Biol. Ecol. 2009, 369, 136–143. [Google Scholar] [CrossRef]
- Pahl, S.L.; Lewis, D.M.; Chen, F.; King, K.D. Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): Effect of some environmental factors. J. Biosci. Bioeng. 2010, 109, 235–239. [Google Scholar] [CrossRef]
- Suman, K.; Kiran, T.; Devi, U.K.; Sarma, N.S. Culture medium optimization and lipid profiling of Cylindrotheca, a lipid- and polyunsaturated fatty acid-rich pennate diatom and potential source of eicosapentaenoic acid. Bot. Mar. 2012, 55, 289–299. [Google Scholar]
- Reis, A.; Gouveia, L.; Veloso, V.; Fernandes, H.L.; Empis, J.A.; Novais, J.M. Eicosapentaenoic acid-rich biomass production by the microalga Phaeodactylum tricornutum in a continuous flow reactor. Bioresour. Technol. 1996, 55, 83–88. [Google Scholar] [CrossRef]
- Alonso, D.L.; Belarbi, E.H.; Fernandez-Sevilla, J.M.; Rodriguez-Ruiz, J.; Grima, E.M. Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochemistry 2000, 54, 461–471. [Google Scholar]
- Zaslavskaia, L.A.; Lippmeier, J.C.; Shih, C.; Ehrhardt, D.; Grossman, A.R.; Apt, K.E. Trophic obligate conversion of an photoautotrophic organism through metabolic engineering. Science 2001, 292, 2073–2075. [Google Scholar] [CrossRef]
- Tan, C.K.; Johns, M.R. Screening of diatoms for heterotrophic eicosapentaenoic acid production. J. Appl. Phycol. 1996, 8, 59–64. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Jiang, Y.; Chen, F. Fatty acid and lipid class composition of the eicosapentaenoic acid-producing microalga, Nitzschia laevis. Food Chem. 2007, 104, 1580–1585. [Google Scholar] [CrossRef]
- Chen, G.-Q; Jiang, Y.; Chen, F. Variation of lipid class composition in Nitzschia laevis as a response to growth temperature change. Food Chem. 2008, 109, 88–94. [Google Scholar] [CrossRef]
- Gimenez, A.G.; Gonzalez, M.J.I.; Medina, A.R.; Grima, E.M.; Salas, S.G.; Cerdan, L.E. Downstream processing and purification of eicosapentaenoic (20:5n-3) and arachidonic acids (20:4n-6) from the microalga Porphyridium cruentum. Bioseparation 1998, 7, 89–99. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Belarbi, E.H.; Rebolloso-Fuentes, M.M. Eicosapentaenoic and arachidonic acids purification from the red microalga Porphyridium cruentum. Bioseparation 2000, 9, 299–306. [Google Scholar] [CrossRef]
- Haimeur, A.; Ulmann, L.; Mimouni, V.; Gueno, F.; Pineau-Vincent, F.; Meskini, N.; Tremblin, G. The role of Odontella aurita, a marine diatom rich in EPA, as a dietary supplement in dyslipidemia, platelet function and oxidative stress in high-fat fed rats. Lipids Health Dis. 2012, 11, 1–13. [Google Scholar] [CrossRef]
- Sijtsma, L.; de Swaaf, M.E. Biotechnological production and applications of the omega-polyunsaturated fatty acid docosahexaenoic acid. Appl. Microbiol. Biot. 2004, 64, 146–153. [Google Scholar] [CrossRef]
- Gu, N.; Lin, Q.; Li, G.; Qin, G.; Lin, J.; Huang, L. Effect of salinity change on biomass and biochemical composition of Nannochloropsis oculata. J. World Aquacult. Soc. 2012, 43, 97–106. [Google Scholar] [CrossRef]
- Raghukumar, S. Thraustochytrid marine protists: Production of PUFAs and other emerging technologies. Mar. Biotechnol. 2008, 10, 631–640. [Google Scholar] [CrossRef]
- Fedorova-Dahms, I.; Marone, P.A.; Bailey-Hall, E.; Ryan, A.S. Safety evaluation of Algal Oil from Schizochytrium sp. Food Chem. Toxicol. 2011, 49, 70–77. [Google Scholar] [CrossRef]
- Fan, K.W.; Chen, F.; Jones, E.B.G.; Vrijmoed, L.L.P. Eicosapentaenoic and docosahexaenoic acids production by and okara-utilizing potential of thraustochytrids. J. Ind. Microbiol. Biot. 2001, 27, 199–202. [Google Scholar] [CrossRef]
- Ethier, S.; Woisard, K.; Vaughan, D.; Wen, Z. Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 2011, 102, 88–93. [Google Scholar] [CrossRef]
- Chi, Z.; Liu, Y.; Frear, C.; Chen, S. Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl. Microbiol. Biot. 2009, 81, 1141–1148. [Google Scholar] [CrossRef]
- Lian, M.; Huang, H.; Ren, L.; Ji, X.; Zhu, J.; Jin, L. Increase of docosahexaenoic acid production by Schizochytrium sp through mutagenesis and enzyme assay. Appl. Biochem. Biotechnol. 2010, 162, 935–941. [Google Scholar] [CrossRef]
- Ren, L.-J.; Ji, X.-J.; Huang, H.; Qu, L.; Feng, Y.; Tong, Q.-Q.; Ouyang, P.K. Development of a stepwise aeration control strategy for efficient docosahexaenoic acid production by Schizochytrium sp. Appl. Microbiol. Biot. 2010, 87, 1649–1656. [Google Scholar] [CrossRef]
- Burja, A.M.; Armenta, R.E.; Radianingtyas, H.; Barrow, C.J. Evaluation of fatty acid extraction methods for Thraustochytrium sp. ONC-T18. J. Agric. Food Chem. 2007, 55, 4795–4801. [Google Scholar]
- Taoka, Y.; Nagano, N.; Okita, Y.; Izumida, H.; Sugimoto, S.; Hayashi, M. Effect of Tween 80 on the growth, lipid accumulation and fatty acid composition of Thraustochytrium aureum ATCC 34304. J. Biosci. Bioeng. 2011, 111, 420–424. [Google Scholar] [CrossRef]
- Quilodran, B.; Hinzpeter, I.; Hormazabal, E.; Quiroz, A.; Shene, C. Docosahexaenoic acid (C22:6n-3, DHA) and astaxanthin production by Thraustochytriidae sp. AS4-A1 a native strain with high similitude to Ulkenia sp.: Evaluation of liquid residues from food industry as nutrient sources. Enzym. Microb. Technol. 2010, 47, 24–30. [Google Scholar]
- Hong, W.-K.; Rairakhwada, D.; Seo, P.-S.; Park, S.-Y.; Hur, B.-K.; Kim, C.H.; Seo, J.W. Production of lipids containing high Levels of docosahexaenoic acid by a newly isolated microalga, Aurantiochytrium sp KRS101. Appl. Biochem. Biotechnol. 2011, 164, 1468–1480. [Google Scholar]
- Pleissner, D.; Eriksen, N.T. Effects of phosphorous, nitrogen, and carbon limitation on biomass composition in batch and continuous flow cultures of the heterotrophic dinoflagellate Crypthecodinium cohnii. Biotechnol. Bioeng. 2012, 109, 2005–2016. [Google Scholar] [CrossRef]
- Da Silva, T.L.; Reis, A. The use of multi-parameter flow cytometry to study the impact of n-dodecane additions to marine dinoflagellate microalga Crypthecodinium cohnii batch fermentations and DHA production. J. Ind. Microbiol. Biot. 2008, 35, 875–887. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F. Effects of medium glucose concentration and pH on docosahexaenoic acid content of heterotrophic Crypthecodinium cohnii. Process Biochem. 2000, 35, 1205–1209. [Google Scholar] [CrossRef]
- Nakahara, T.; Yokochi, T.; Higashihara, T.; Tanaka, S.; Yaguchi, T.; Honda, D. Production of docosahexaenoic and docosapentaenoic acids by Schizochytrium sp. isolated from Yap islands. J. Am. Oil Chem. Soc. 1996, 73, 1421–1426. [Google Scholar] [CrossRef]
- Yokochi, T.; Honda, D.; Higashihara, T.; Nakahara, T. Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Appl. Microbiol. Biot. 1998, 49, 72–76. [Google Scholar] [CrossRef]
- Yaguchi, T.; Tanaka, S.; Yokochi, T.; Nakahara, T.; Higashihara, T. Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21. J. Am. Oil Chem. Soc. 1997, 74, 1431–1434. [Google Scholar] [CrossRef]
- Morita, E.; Kumon, Y.; Nakahara, T.; Kagiwada, S.; Noguchi, T. Docosahexaenoic acid production and lipid-body formation in Schizochytrium limacinum SR21. Mar. Biotechnol. 2006, 8, 319–327. [Google Scholar] [CrossRef]
- Fan, K.-W.; Jiang, Y.; Faan, Y.-W.; Chen, F. Lipid characterization of mangrove thraustochytrid—Schizochytrium mangrovei. J. Agric. Food Chem. 2007, 55, 2906–2910. [Google Scholar] [CrossRef]
- Jeh, E.-J.; Kumaran, R.S.; Hur, B.-K. Lipid body formation by Thraustochytrium aureum (ATCC 34304) in response to cell age. Korean J. Chem. Eng. 2008, 25, 1103–1109. [Google Scholar] [CrossRef]
- Huang, J.Z.; Aki, T.; Hachida, K.; Yokochi, T.; Kawamoto, S.; Shigeta, S.; Ono, K.; Suzuki, O. Profile of polyunsaturated fatty acids produced by Thraustochytrium sp KK17-3. J. Am. Oil Chem. Soc. 2001, 78, 605–610. [Google Scholar] [CrossRef]
- FDA, Food and Drug Administration, GRAS Notice for Ulkenia DHA Oil Derived from Ulkenia sp. Microalga; Food and Drug Administration: Silver Spring, MD, USA, 2010.
- Fan, K.W.; Chen, F. Production of High-Value Products by Marine Microalgae Thraustochytrids. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Innis, S.M.; Hansen, J.W. Plasma fatty acid responses, metabolic effects, and safety of microalgal and fungal oils rich in arachidonic and docosahexaenoic acids in healthy adults. Am. J. Clin. Nutr. 1996, 64, 159–167. [Google Scholar]
- Tam, P.S.Y.; Umeda-Sawada, R.; Yaguchi, T.; Akimoto, K.; Kiso, Y.; Igarashi, O. The metabolism and distribution of docosapentaenoic acid (n-6) in rats and rat hepatocytes. Lipids 2000, 35, 71–75. [Google Scholar] [CrossRef]
- Ratledge, C.; Kanagachandran, K.; Anderson, A.J.; Grantham, D.J.; Stephenson, J.C. Production of docosahexaenoic acid by Crypthecodinium cohnii grown in a pH-auxostat culture with acetic acid as principal carbon source. Lipids 2001, 36, 1241–1246. [Google Scholar] [CrossRef]
- Mendes, A.; Guerra, P.; Madeira, V.; Ruano, F.; da Silva, T.L.; Reis, A. Study of docosahexaenoic acid production by the heterotrophic microalga Crypthecodinium cohnii CCMP 316 using carob pulp as a promising carbon source. World J. Microb. Biot. 2007, 23, 1209–1215. [Google Scholar] [CrossRef]
- Couto, R.M.; Simoes, P.C.; Reis, A.; da Silva, T.L.; Martins, V.H.; Sanchez-Vicente, Y. Supercritical fluid extraction of lipids from the heterotrophic microalga Crypthecodinium cohnii. Eng. Life Sci. 2010, 10, 158–164. [Google Scholar]
- Mendes, A.; Reis, A.; Vasconcelos, R.; Guerra, P.; da Silva, T.L. Crypthecodinium cohnii with emphasis on DHA production: A review. J. Appl. Phycol. 2009, 21, 199–214. [Google Scholar] [CrossRef]
- Ratledge, C. Single cell oils—Have they a biotechnological future. Trends Biotechnol. 1993, 11, 278–284. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef]
- Brondz, I. Development of fatty acid analysis by high-performance liquid chromatography, gas chromatography, and related techniques. Anal. Chim. Acta 2002, 465, 1–37. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Malcata, F.X. Preparation of fatty acid methyl esters for gas-chromatographic analysis of marine lipids: Insight studies. J. Agric. Food Chem. 2005, 53, 5049–5059. [Google Scholar]
- Wichard, T.; Gerecht, A.; Boersma, M.; Poulet, S.A.; Wiltshire, K.; Pohnert, G. Lipid and fatty acid composition of diatoms revisited: Rapid wound-activated change of food quality parameters influences herbivorous copepod reproductive success. ChemBioChem 2007, 8, 1146–1153. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C.; Dangour, A.D.; Diekman, C.; Eilander, A.; Koletzko, B.; Meijer, G.W.; Mozaffarian, D.; Niinikoski, H.; Osendarp, S.J.; Pietinen, P.; et al. Essential fats for future health. Eur. J. Clin. Nutr. 2010, 64, 1–13. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martins, D.A.; Custódio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K.M. Alternative Sources of n-3 Long-Chain Polyunsaturated Fatty Acids in Marine Microalgae. Mar. Drugs 2013, 11, 2259-2281. https://doi.org/10.3390/md11072259
Martins DA, Custódio L, Barreira L, Pereira H, Ben-Hamadou R, Varela J, Abu-Salah KM. Alternative Sources of n-3 Long-Chain Polyunsaturated Fatty Acids in Marine Microalgae. Marine Drugs. 2013; 11(7):2259-2281. https://doi.org/10.3390/md11072259
Chicago/Turabian StyleMartins, Dulce Alves, Luísa Custódio, Luísa Barreira, Hugo Pereira, Radhouan Ben-Hamadou, João Varela, and Khalid M. Abu-Salah. 2013. "Alternative Sources of n-3 Long-Chain Polyunsaturated Fatty Acids in Marine Microalgae" Marine Drugs 11, no. 7: 2259-2281. https://doi.org/10.3390/md11072259





