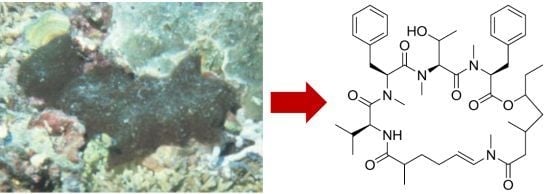
Bouillonamide: A Mixed Polyketide–Peptide Cytotoxin from the Marine Cyanobacterium Moorea bouillonii
Abstract
:1. Introduction


2. Results and Discussion
| Unit | Position | δH, mult. (J in Hz) | δC | HMBC |
|---|---|---|---|---|
| Mmaha | 1 | 175.7 s | ||
| 2 | 2.34 m | 42.3 d | C-1, C-7 | |
| 3 | 1.68 m | 36.9 t | ||
| 1.27 m | ||||
| 4 | 1.93 m | 29.3 t | ||
| 1.69 m | ||||
| 5 | 4.89 m | 109.6 d | ||
| 6 | 6.64 d (13.4) | 130.5 d | C-4, C-5, C-8, C-9 | |
| 7 | 1.19 d (7.1) | 20.5 q | C-1, C-2, C-3 | |
| 8 NMe | 3.08 s | 29.7 q | C-5, C-6, C-9 | |
| Mhha | 9 | 171.3 s | ||
| 10 | 2.44 m | 39.5 t | C-9, C-11, C-12, C-16 | |
| 2.44 m | C-9, C-11, C-12, C-16 | |||
| 11 | 2.03 m | 28.0 d | ||
| 12 | 1.64 m | 40.5 t | C-10, C-1, C-13, C-14, C-15, C-16 | |
| 1.52 m | C-10, C-1, C-13, C-14, C-16 | |||
| 13 | 4.96 m | 76.6 d | C-15 | |
| 14 | 1.85 m | 25.9 t | ||
| 1.62 m | ||||
| 15 | 0.94 t (7.4) | 9.2 q | C-13, C-14 | |
| 16 | 0.94 m | 20.6 q | C-10, C-11, C-12 | |
| NMe-Phe-1 | 17 | 169.5 s | ||
| 18 | 4.31 dd (11.5, 3.8) | 61.4 d | C-17, C-19, C-26, C-27 | |
| 19 | 3.28 m | 36.1 t | C-18, C-20, C-21/25 | |
| 2.90 m | C-18, C-20, C-21/25 | |||
| 20 | 136.5 s | |||
| 21/25 | 7.13 m | 129.5 d | ||
| 22/24 | 7.35 m | 128.8 d | ||
| 23 | 7.06 m | 127.4 d | ||
| 26 NMe | 2.91 s | 30.9 q | C-18, C-27 | |
| NMe-Thr | 27 | 171.3 s | ||
| 28 | 4.54 d (4.7) | 57.7 d | C-29, C-30, C-31 | |
| 29 | 3.85 m | 67.8 d | ||
| OH | 3.34 d (4.7) | C-29, C-30 | ||
| 30 | 0.84 d (6.2) | 19.9 q | C-28, C-29 | |
| 31 NMe | 2.79 s | 32.8 q | C-32, C-28 | |
| NMe-Phe-2 | 32 | 170.4 s | ||
| 33 | 5.49 dd (8.4, 5.6) | 56.4 d | C-32, C-34, C-41, C-42 | |
| 34 | 3.28 m | 35.3 t | C-32, C-33, C-35, C-36/40, C-42 | |
| 2.90 m | C-32, C-33, C-35, C-36/40, C-42 | |||
| 35 | 138.0 s | |||
| 36/40 | 7.23 m | 129 5 d | ||
| 37/39 | 7.35 m | 129.3 d | ||
| 38 | 7.24 m | 127.7 d | ||
| 41 NMe | 3.10 s | 31.3 q | C-33, C-42 | |
| Val | 42 | 172.1 s | ||
| 43 | 4.86 dd (8.6, 3.0) | 52.8 d | C-42, C-44, C-45, C-46 | |
| 44 | 1.96 m | 31.2 d | ||
| 45 | 0.74 d (6.7) | 16.4 q | C-43, C-44, C-46 | |
| 46 | 0.95 d (6.8) | 20.6 q | C-43, C-44, C-45 | |
| NH | 6.02 d (8.6) | C-42, C-1 |

3. Experimental Section
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation of Bouillonamide (1)
3.4. Acid Hydrolysis and Marfey’s Analysis of Bouillonamide (1)
3.5. Brine Shrimp Toxicity and Cytotoxicity Assays
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gerwick, W.H.; Tan, L.T.; Sitachitta, N. Nitrogen-Containing Metabolites from Marine Cyanobacteria. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: San Diego, CA, USA, 2001; Volume 57, pp. 75–184. [Google Scholar]
- Tan, L.T. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar] [CrossRef]
- Tan, L.T. Marine Cyanobacteria: A Treasure Trove of Bioactive Secondary Metabolites for Drug Discovery. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 36, pp. 67–110. [Google Scholar]
- Minich, S.S. Brentuximab vedotin: A new age in the treatment of Hodgkin lymphoma and anaplastic large cell lymphoma. Ann. Pharmacother. 2012, 46, 377–383. [Google Scholar] [CrossRef]
- Hoffmann, L.; Demoulin, V. Marine cyanophyceae of Papua New Guinea. II. Lyngbya bouillonii sp. nov., a remarkable tropical reef-inhabiting blue-green alga. Belg. J. Bot. 1991, 124, 82–88. [Google Scholar]
- Engene, N.; Rottacker, E.C.; Kastovsky, J.; Byrum, T.; Choi, H.; Ellisman, M.H.; Komarek, J.; Gerwick, W.H. Moorea producens gen. nov., sp. nov. and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62, 1171–1178. [Google Scholar] [CrossRef]
- Klein, D.; Braekman, J.C.; Daloze, D.; Hoffinann, L.; Castillo, G.; Demoulin, V.V. Madangolide and laingolide A, two novel macrolides from Lyngbya bouillonii (Cyanobacteria). J. Nat. Prod. 1999, 62, 934–936. [Google Scholar] [CrossRef]
- Pereira, A.R.; McCue, C.F.; Gerwick, W.H. Cyanolide A, a glycosidic macrolide with potent molluscicidal activity from the Papua New Guinea cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2010, 73, 217–220. [Google Scholar] [CrossRef]
- Matthew, S.; Schupp, P.J.; Luesch, H. Apratoxin E, a cytotoxic peptolide from a guamanian collection of the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2008, 71, 1113–1116. [Google Scholar] [CrossRef]
- Tidgewell, K.; Engene, N.; Byrum, T.; Media, J.; Doi, T.; Valeriote, F.A.; Gerwick, W.H. Evolved diversification of a modular natural product pathway: Apratoxins F and G, two cytotoxic cyclic depsipeptides from a Palmyra collection of Lyngbya bouillonii. ChemBioChem 2010, 11, 1458–1466. [Google Scholar] [CrossRef]
- Soria-Mercado, I.E.; Pereira, A.; Cao, Z.; Murray, T.F.; Gerwick, W.H. Alotamide A, a novel neuropharmacological agent from the marine cyanobacterium Lyngbya bouillonii. Org. Lett. 2009, 11, 4704–4707. [Google Scholar] [CrossRef]
- Choi, H.; Mevers, E.; Byrum, T.; Valeriote, F.A.; Gerwick, W.H. Lyngbyabellins K–N from Two Palmyra Atoll Collections of the Marine Cyanobacterium Moorea bouillonii. Eur. J. Org. Chem. 2012, 2012, 5141–5150. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Yakovleva, I.M.; Titlyanova, T.V. Interaction between benthic algae (Lyngbya bouillonii, Dictyota dichotoma) and scleractinian coral Porites lutea in direct contact. J. Exp. Mar. Biol. Ecol. 2007, 342, 282–291. [Google Scholar] [CrossRef]
- Tan, L.T.; Marquez, B.L.; Gerwick, W.H. Lyngbouilloside, a novel glycosidic macrolide from the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2002, 65, 925–928. [Google Scholar] [CrossRef]
- Marfey, P. Determination of d-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res. Commun. 1984, 49, 591–596. [Google Scholar] [CrossRef]
- Carmeli, S.; Moore, R.E.; Patterson, G.M.L.; Yoshida, W.Y. Biosynthesis of tolytoxin. Origin of the carbons and heteroatoms. Tetrahedron Lett. 1993, 34, 5571–5574. [Google Scholar] [CrossRef]
- Klein, D.; Braekman, J.C.; Daloze, D.; Hoffmann, L.; Demoulin, V. Laingolide, a novel 15-membered macrolide from Lyngbya bouillonii (Cyanophyceae). Tetrahedron Lett. 1996, 37, 7519–7520. [Google Scholar] [CrossRef]
- Smith, C.D.; Carmeli, S.; Moore, R.E.; Patterson, G.M. Scytophycins, novel microfilament-depolymerizing agents which circumvent P-glycoprotein-mediated multidrug resistance. Cancer Res. 1993, 53, 1343–1347. [Google Scholar]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Corbett, T.H. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 2001, 123, 5418–5423. [Google Scholar]
- Luesch, H.; Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Ulongamides A–F, new β-amino acid-containing cyclodepsipeptides from Palauan collections of the marine cyanobacterium Lyngbya sp. J. Nat. Prod. 2002, 65, 996–1000. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tan, L.T.; Okino, T.; Gerwick, W.H. Bouillonamide: A Mixed Polyketide–Peptide Cytotoxin from the Marine Cyanobacterium Moorea bouillonii. Mar. Drugs 2013, 11, 3015-3024. https://doi.org/10.3390/md11083015
Tan LT, Okino T, Gerwick WH. Bouillonamide: A Mixed Polyketide–Peptide Cytotoxin from the Marine Cyanobacterium Moorea bouillonii. Marine Drugs. 2013; 11(8):3015-3024. https://doi.org/10.3390/md11083015
Chicago/Turabian StyleTan, Lik Tong, Tatsufumi Okino, and William H. Gerwick. 2013. "Bouillonamide: A Mixed Polyketide–Peptide Cytotoxin from the Marine Cyanobacterium Moorea bouillonii" Marine Drugs 11, no. 8: 3015-3024. https://doi.org/10.3390/md11083015