Towards the Small and the Beautiful: A Small Dibromotyrosine Derivative from Pseudoceratina sp. Sponge Exhibits Potent Apoptotic Effect through Targeting IKK/NFκB Signaling Pathway
Abstract
:1. Introduction
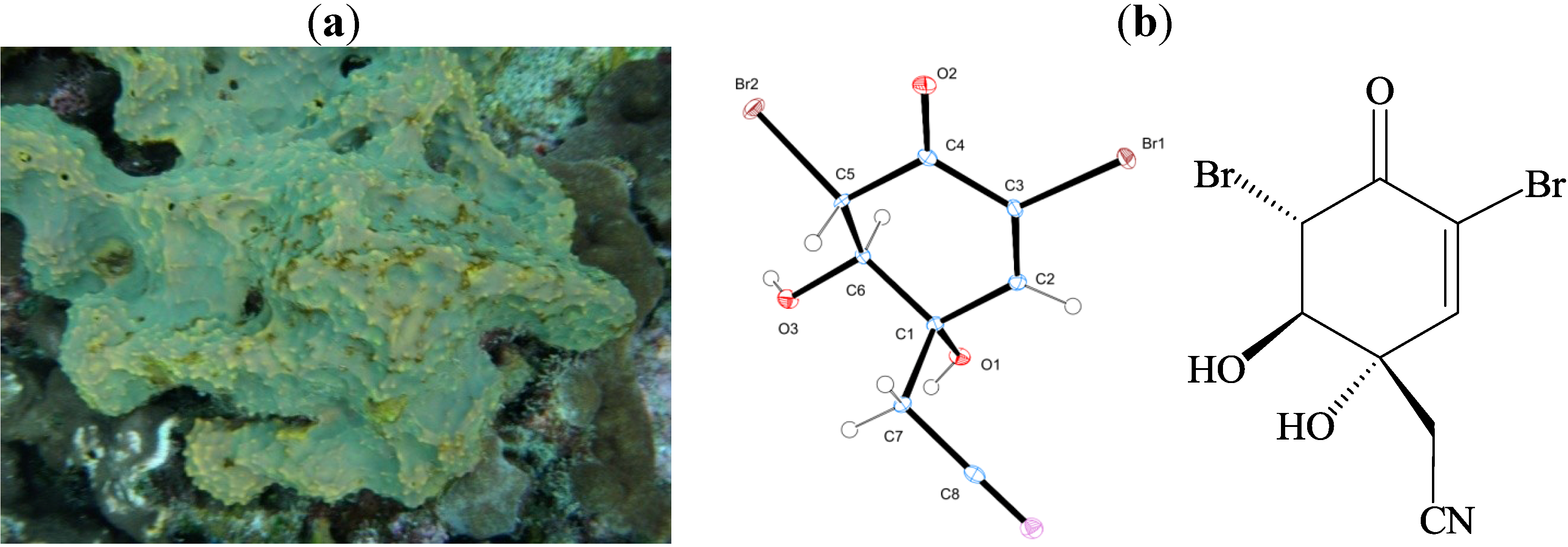
2. Results
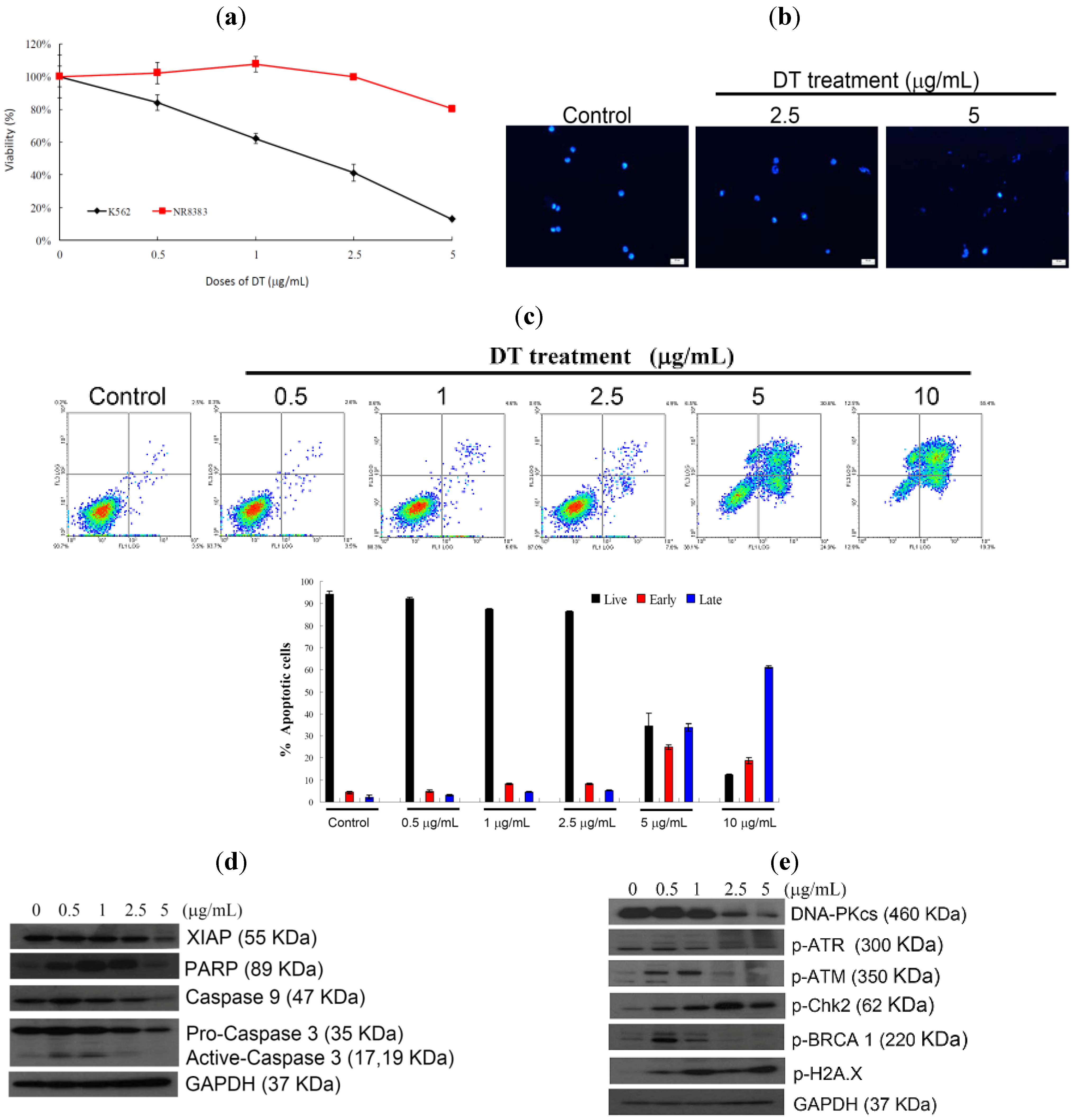
2.1. DT Inhibits Cellular Growth and Induces Apoptosis in K562 Cells
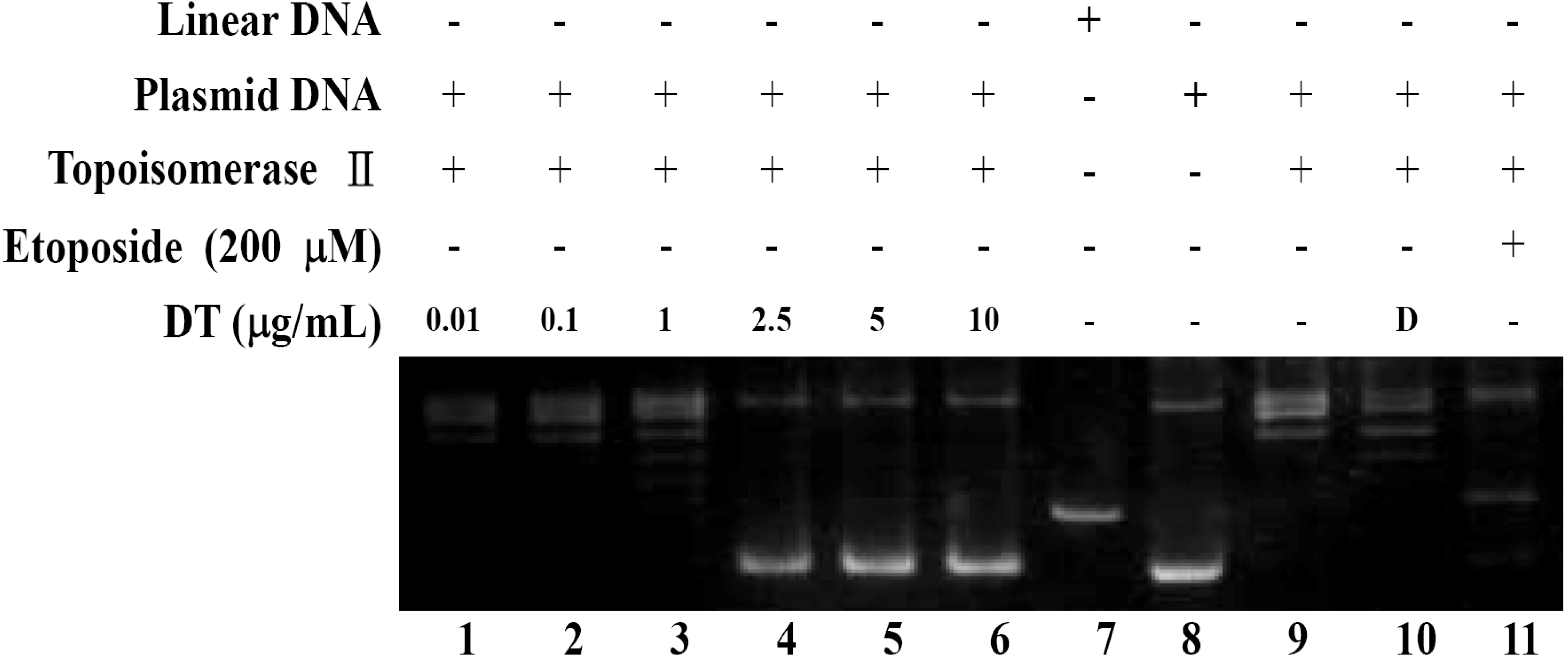
2.2. DT Inhibits Topoisomerase II Catalytic Cycle
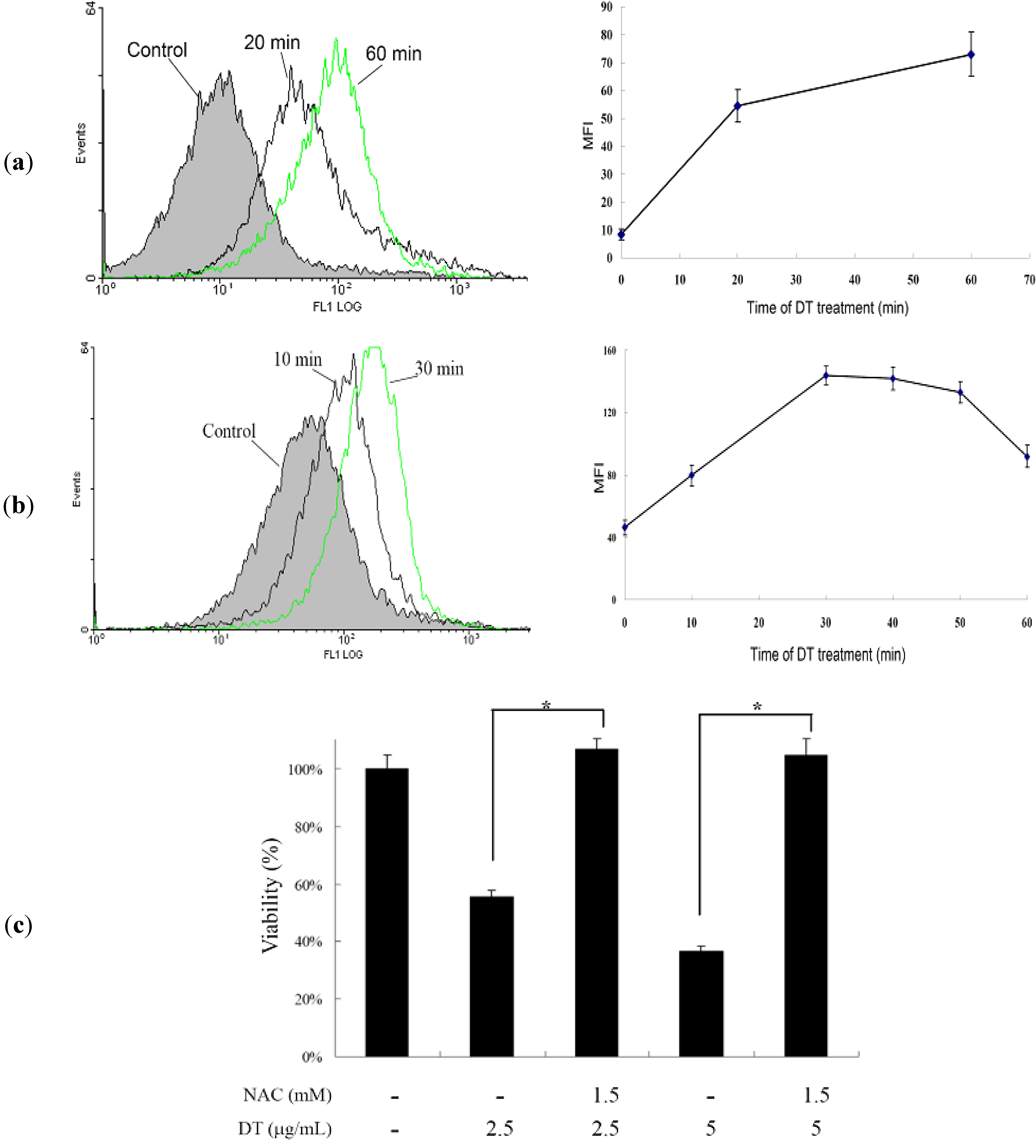
2.3. DT-Induced Apoptosis Is Mediated through Reactive Oxygen Species (ROS) Generation in K562 Cells
2.4. DT-Induced Oxidative Stress Disturbs Mitochondrial Membrane Potential (MMP)
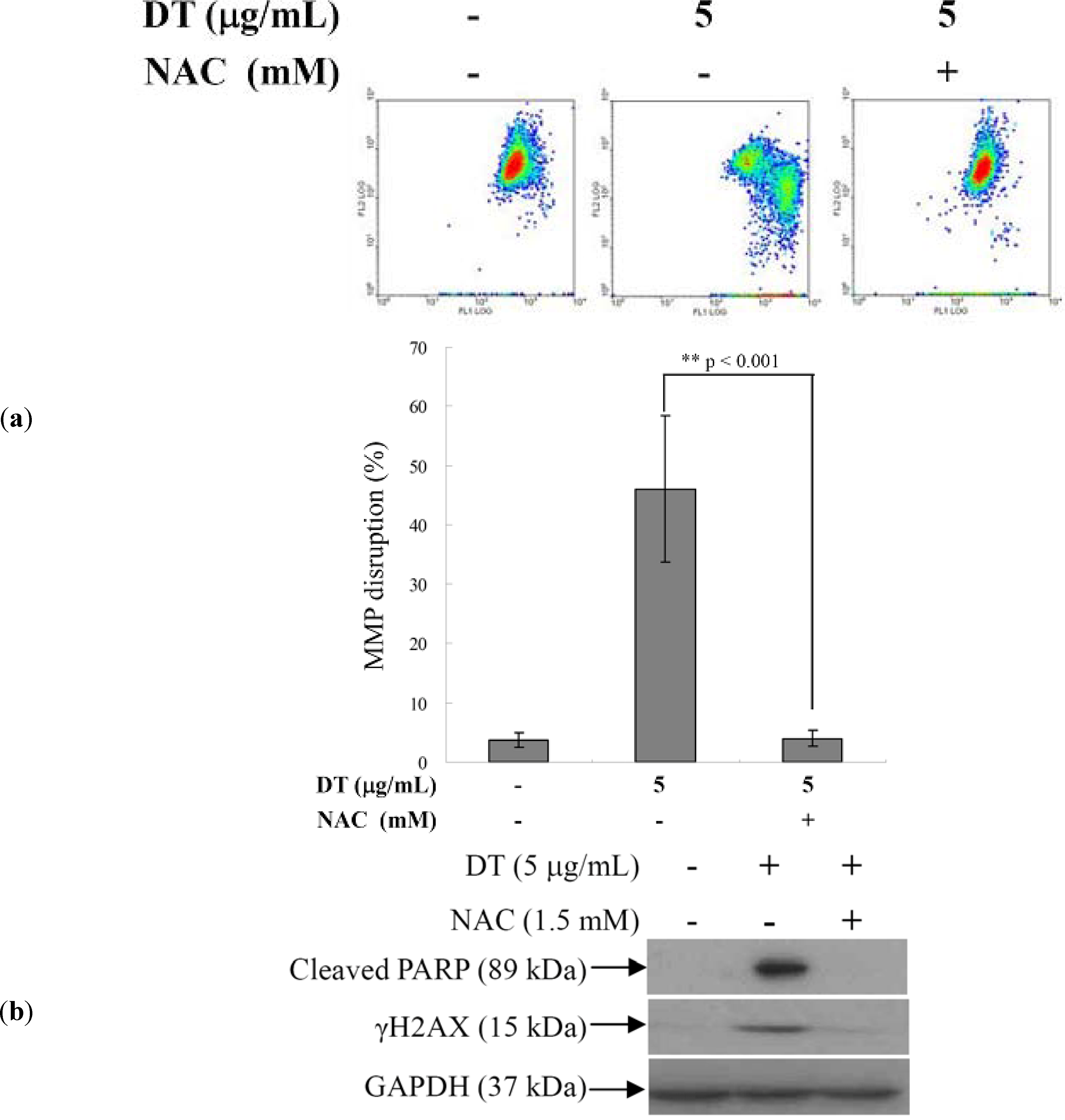
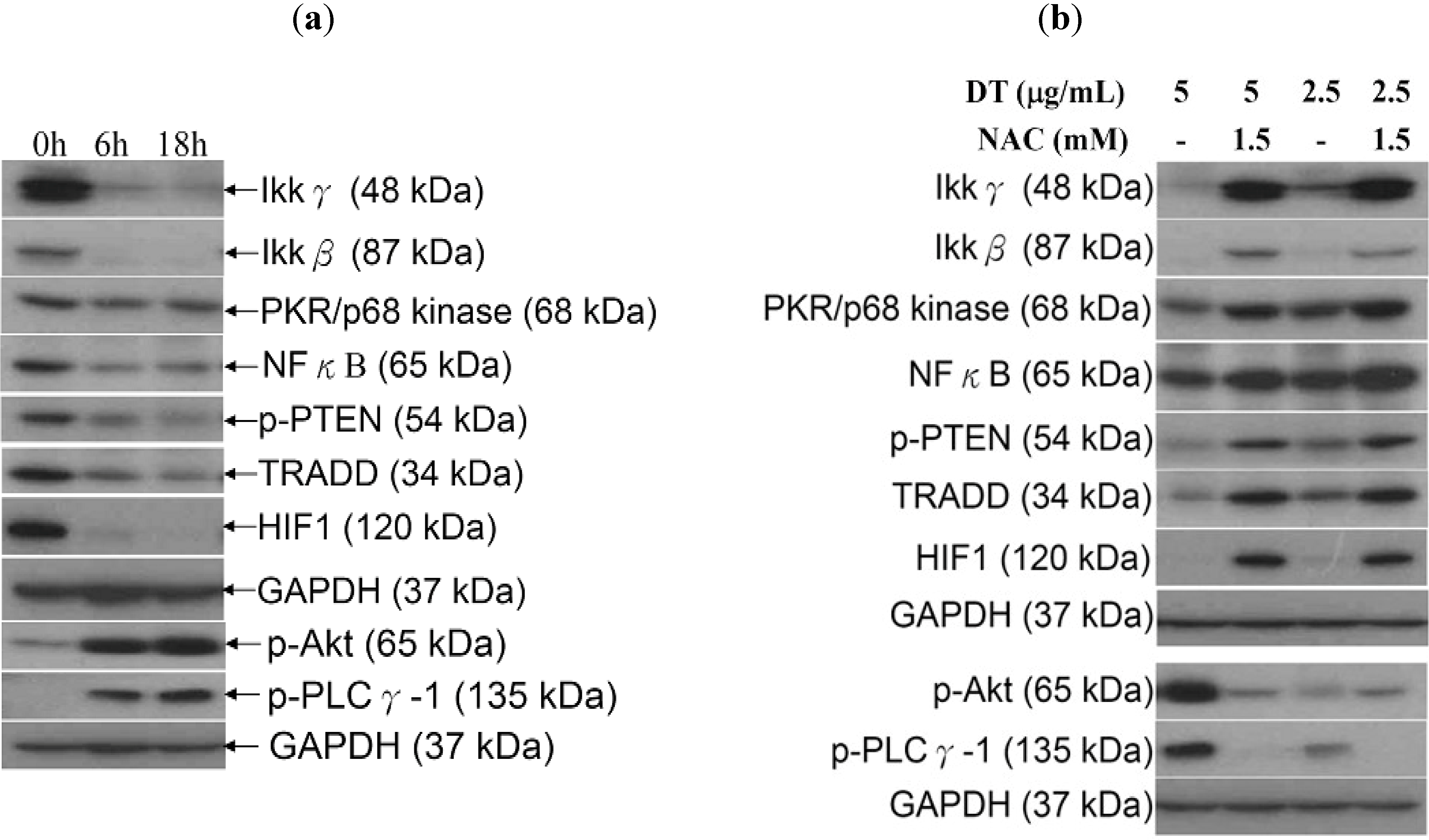
2.5. ROS Generation in DT-Induced Apoptosis is Mediated through the Inhibition of IKK/NFκB and the Activation of PI3K/Akt Pathways
3. Discussion
4. Experimental Section
4.1. Bioassays Materials
4.2. Preparation of (1′R,5′S,6′S)-2-(3′,5′-dibromo-1′,6′-dihydroxy-4′-oxocyclohex-2′-enyl) Acetonitrile (DT) Stock Solution
4.3. MTT Antiproliferative Assay
4.4. Annexin V/PI Apoptosis Assay
4.5. Determination of ROS Generation and MMP Disruption
4.6. Assay of Topoisomerase II Inhibitors and Poisons
4.7. Western Blotting Analysis
4.8. Statistics
5. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Kunze, K.; Niemann, H.; Ueberlein, S.; Schulze, R.; Ehrlich, H.; Brunner, E.; Proksch, P.; Pee, K.H. Brominated skeletal components of the marine demosponges, Aplysina cavernicola and Ianthella basta: Analytical and biochemical investigations. Mar. Drugs 2013, 11, 1271–1287. [Google Scholar] [CrossRef]
- Ebada, S.S.; Lin, W.; Proksch, P. Bioactive sesterterpenes and triterpenes from marine sponges: Occurrence and pharmacological significance. Mar. Drugs 2010, 8, 313–346. [Google Scholar] [CrossRef]
- Lira, N.S.; Montes, R.C.; Tavares, J.F.; da Silva, M.S.; da Cunha, E.V.; de Athayde-Filho, P.F.; Rodrigues, L.C.; da Silva Dias, C.; Barbosa-Filho, J.M. Brominated compounds from marine sponges of the genus Aplysina and a compilation of their 13C NMR spectral data. Mar. Drugs 2011, 9, 2316–2368. [Google Scholar] [CrossRef]
- Isidori, A. Clinical experimental evaluation of the effect of dibromotyrosine on thyroid function. Int. J. Clin. Pharmacol. Biopharm. 1978, 16, 180–182. [Google Scholar]
- Galeano, E.; Thomas, O.P.; Robledo, S.; Munoz, D.; Martinez, A. Antiparasitic bromotyrosine derivatives from the marine sponge Verongula rigida. Mar. Drugs 2011, 9, 1902–1913. [Google Scholar] [CrossRef]
- Hinterding, K.; Knebel, A.; Herrlich, P.; Waldmann, H. Synthesis and biological evaluation of aeroplysinin analogues: A new class of receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. 1998, 6, 1153–1162. [Google Scholar]
- Cordoba, R.; Tormo, N.S.; Medarde, A.F.; Plumet, J. Antiangiogenic versus cytotoxic activity in analogues of aeroplysinin-1. Bioorg. Med. Chem. 2007, 15, 5300–5315. [Google Scholar] [CrossRef]
- Martinez-Poveda, B.; Garcia-Vilas, J.A.; Cardenas, C.; Melgarejo, E.; Quesada, A.R.; Medina, M.A. The brominated compound aeroplysinin-1 inhibits proliferation and the expression of key pro-inflammatory molecules in human endothelial and monocyte cells. PLoS One 2013, 8, e55203. [Google Scholar]
- Teeyapant, R.; Woerdenbag, H.J.; Kreis, P.; Hacker, J.; Wray, V.; Witte, L.; Proksch, P. Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba. Z. Naturforsch C 1993, 48, 939–945. [Google Scholar]
- Martinez-Poveda, B.; Rodriguez-Nieto, S.; Garcia-Caballero, M.; Medina, M.A.; Quesada, A.R. The antiangiogenic compound aeroplysinin-1 induces apoptosis in endothelial cells by activating the mitochondrial pathway. Mar. Drugs 2012, 10, 2033–2046. [Google Scholar] [CrossRef]
- Sallam, A.A.; Ramasahayam, S.; Meyer, S.A.; El Sayed, K.A. Design, synthesis, and biological evaluation of dibromotyrosine analogues inspired by marine natural products as inhibitors of human prostate cancer proliferation, invasion, and migration. Bioorg. Med. Chem. 2010, 18, 7446–7457. [Google Scholar] [CrossRef]
- Kalaitzis, J.A.; Davis, R.A.; Quinn, R.J. Unequivocal 13C NMR assignment of cyclohexadienyl rings in bromotyrosine-derived metabolites from marine sponges. Magn. Reson. Chem. 2012, 50, 749–754. [Google Scholar] [CrossRef]
- Hillgren, J.M.; Oberg, C.T.; Elofsson, M. Syntheses of pseudoceramines A–D and a new synthesis of spermatinamine, bromotyrosine natural products from marine sponges. Org. Biomol. Chem. 2012, 10, 1246–1254. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Fechner, G.A.; Boyle, A.; Simpson, M.; Addepalli, R.; Avery, V.M.; Hooper, J.N.; Cheung, T.; Chen, H.; et al. Aplysamine 6, an alkaloidal inhibitor of isoprenylcysteine carboxyl methyltransferase from the sponge Pseudoceratina sp. J. Nat. Prod. 2008, 71, 1066–1067. [Google Scholar] [CrossRef]
- Kon, Y.; Kubota, T.; Shibazaki, A.; Gonoi, T.; Kobayashi, J. Ceratinadins A–C, new bromotyrosine alkaloids from an Okinawan marine sponge Pseudoceratina sp. Bioorg. Med. Chem. Lett. 2010, 20, 4569–4572. [Google Scholar] [CrossRef]
- Lebouvier, N.; Jullian, V.; Desvignes, I.; Maurel, S.; Parenty, A.; Dorin-Semblat, D.; Doerig, C.; Sauvain, M.; Laurent, D. Antiplasmodial activities of homogentisic acid derivative protein kinase inhibitors isolated from a Vanuatu marine sponge Pseudoceratina sp. Mar. Drugs 2009, 7, 640–653. [Google Scholar] [CrossRef]
- Herter-Sprie, G.S.; Chen, S.; Hopker, K.; Reinhardt, H.C. Synthetic lethality as a new concept for the treatment of cancer. Ger. Med. Wkly. 2011, 136, 1526–1530. [Google Scholar]
- Du, Y.C.; Chang, F.R.; Wu, T.Y.; Hsu, Y.M.; El-Shazly, M.; Chen, C.F.; Sung, P.J.; Lin, Y.Y.; Lin, Y.H.; Wu, Y.C.; et al. Antileukemia component, dehydroeburicoic acid from Antrodia camphorata induces DNA damage and apoptosis in vitro and in vivo models. Phytomedicine 2012, 19, 788–796. [Google Scholar] [CrossRef]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Hutti, J.E.; Pfefferle, A.D.; Russell, S.C.; Sircar, M.; Perou, C.M.; Baldwin, A.S. Oncogenic PI3K mutations lead to NF-kappaB-dependent cytokine expression following growth factor deprivation. Cancer Res. 2012, 72, 3260–3269. [Google Scholar] [CrossRef]
- Kim, H.J.; Hawke, N.; Baldwin, A.S. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006, 13, 738–747. [Google Scholar] [CrossRef]
- Li, X.; Abdel-Mageed, A.B.; Mondal, D.; Kandil, E. The nuclear factor kappa-B signaling pathway as a therapeutic target against thyroid cancers. Thyroid 2013, 23, 209–218. [Google Scholar] [CrossRef]
- Lampiasi, N.; Azzolina, A.; Umezawa, K.; Montalto, G.; McCubrey, J.A.; Cervello, M. The novel NF-kappaB inhibitor DHMEQ synergizes with celecoxib to exert antitumor effects on human liver cancer cells by a ROS-dependent mechanism. Cancer Lett. 2012, 322, 35–44. [Google Scholar] [CrossRef]
- Madonna, G.; Ullman, C.D.; Gentilcore, G.; Palmieri, G.; Ascierto, P.A. NF-kappaB as potential target in the treatment of melanoma. J. Transl. Med. 2012, 10, 53. [Google Scholar] [CrossRef]
- Martelli, A.M.; Tabellini, G.; Bressanin, D.; Ognibene, A.; Goto, K.; Cocco, L.; Evangelisti, C. The emerging multiple roles of nuclear Akt. Biochim. Biophys. Acta 2012, 1823, 2168–2178. [Google Scholar] [CrossRef]
- Sawyers, C.L. Chronic myeloid leukemia. N. Engl. J. Med. 1999, 340, 1330–1340. [Google Scholar] [CrossRef]
- Rowley, J.D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef]
- Druker, B.J.; Guilhot, F.; O’Brien, S.G.; Gathmann, I.; Kantarjian, H.; Gattermann, N.; Deininger, M.W.; Silver, R.T.; Goldman, J.M.; Stone, R.M.; et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006, 355, 2408–2417. [Google Scholar] [CrossRef]
- Ibrahim, A.R.; Eliasson, L.; Apperley, J.F.; Milojkovic, D.; Bua, M.; Szydlo, R.; Mahon, F.X.; Kozlowski, K.; Paliompeis, C.; Foroni, L.; et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood 2011, 117, 3733–3736. [Google Scholar] [CrossRef]
- Georgiades, S.N.; Clardy, J. Total synthesis of psammaplysenes A and B, naturally occurring inhibitors of FOXO1a nuclear export. Org. Lett. 2005, 7, 4091–4094. [Google Scholar] [CrossRef]
- Myatt, S.S.; Brosens, J.J.; Lam, E.W. Sense and sensitivity: FOXO and ROS in cancer development and treatment. Antioxid. Redox Signal. 2011, 14, 675–687. [Google Scholar] [CrossRef]
- Greer, E.L.; Brunet, A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005, 24, 7410–7425. [Google Scholar] [CrossRef]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef]
- Downward, J. PI 3-kinase, Akt and cell survival. Semin. Cell Dev. Biol. 2004, 15, 177–182. [Google Scholar] [CrossRef]
- Blanco-Aparicio, C.; Renner, O.; Leal, J.F.; Carnero, A. PTEN, more than the AKT pathway. Carcinogenesis 2007, 28, 1379–1386. [Google Scholar] [CrossRef]
- Ahn, J.; Won, M.; Choi, J.H.; Kim, Y.S.; Jung, C.R.; Im, D.S.; Kyun, M.L.; Lee, K.; Song, K.B.; Chung, K.S. Reactive oxygen species-mediated activation of the Akt/ASK1/p38 signaling cascade and p21 downregulation are required for shikonin-induced apoptosis. Apoptosis 2013, 18, 870–881. [Google Scholar] [CrossRef]
- Szanto, A.; Hellebrand, E.E.; Bognar, Z.; Tucsek, Z.; Szabo, A.; Gallyas, F., Jr.; Sumegi, B.; Varbiro, G. PARP-1 inhibition-induced activation of PI-3-kinase-Akt pathway promotes resistance to taxol. Biochem. Pharmacol. 2009, 77, 1348–1357. [Google Scholar] [CrossRef]
- Xing, H.; Weng, D.; Chen, G.; Tao, W.; Zhu, T.; Yang, X.; Meng, L.; Wang, S.; Lu, Y.; Ma, D. Activation of fibronectin/PI-3K/Akt2 leads to chemoresistance to docetaxel by regulating survivin protein expression in ovarian and breast cancer cells. Cancer Lett. 2008, 261, 108–119. [Google Scholar] [CrossRef]
- Ziegelbauer, K.; Gantner, F.; Lukacs, N.W.; Berlin, A.; Fuchikami, K.; Niki, T.; Sakai, K.; Inbe, H.; Takeshita, K.; Ishimori, M.; Komura, H.; Murata, T.; Lowinger, T.; Bacon, K.B. A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br. J. Pharmacol. 2005, 145, 178–192. [Google Scholar] [CrossRef]
- Castro, A.C.; Dang, L.C.; Soucy, F.; Grenier, L.; Mazdiyasni, H.; Hottelet, M.; Parent, L.; Pien, C.; Palombella, V.; Adams, J. Novel IKK inhibitors: Beta-Carbolines. Bioorg. Med. Chem. Lett. 2003, 13, 2419–2422. [Google Scholar] [CrossRef]
- Kishore, N.; Sommers, C.; Mathialagan, S.; Guzova, J.; Yao, M.; Hauser, S.; Huynh, K.; Bonar, S.; Mielke, C.; Albee, L.; et al. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J. Biol. Chem. 2003, 278, 32861–32871. [Google Scholar] [CrossRef]
- Christopher, J.A.; Avitabile, B.G.; Bamborough, P.; Champigny, A.C.; Cutler, G.J.; Dyos, S.L.; Grace, K.G.; Kerns, J.K.; Kitson, J.D.; Mellor, G.W.; et al. The discovery of 2-amino-3,5-diarylbenzamide inhibitors of IKK-alpha and IKK-beta kinases. Bioorg. Med. Chem. Lett. 2007, 17, 3972–3977. [Google Scholar] [CrossRef]
- Gamble, C.; McIntosh, K.; Scott, R.; Ho, K.H.; Plevin, R.; Paul, A. Inhibitory kappa B Kinases as targets for pharmacological regulation. Br. J. Pharmacol. 2012, 165, 802–819. [Google Scholar] [CrossRef]
- Nam, S.Y.; Ko, Y.S.; Jung, J.; Yoon, J.; Kim, Y.H.; Choi, Y.J.; Park, J.W.; Chang, M.S.; Kim, W.H.; Lee, B.L. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-kappa B promotes gastric tumour growth and angiogenesis. Br. J. Cancer 2011, 104, 166–174. [Google Scholar] [CrossRef]
- Kuphal, S.; Winklmeier, A.; Warnecke, C.; Bosserhoff, A.K. Constitutive HIF-1 activity in malignant melanoma. Eur. J. Cancer 2010, 46, 1159–1169. [Google Scholar] [CrossRef]
- Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre (deposition number CCDC 930829). Copies of the data can be obtained, f.o.c., on application to the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44-1223-336033 or E-Mail: [email protected]).
- Capon, R.; Macleod, J. Two epimeric dibromo nitriles from the australian sponge Aplysina laevis. Aust. J. Chem. 1987, 40, 341–346. [Google Scholar] [CrossRef]
- Santalova, E.A.; Denisenko, V.A.; Glazunov, V.P.; Kalinovskii, A.I.; Anastyuk, S.D.; Stonik, V.A. Dibromotyrosine derivatives from the ethanol extract of the marine sponge Aplysina sp.: Structures, transformations and origin. Russian Chem. Bull. 2100, 60, 570–580. [Google Scholar]
- Huang, K.-J.; Chen, Y.-C.; El-Shazly, M.; Du, Y.-C.; Su, J.-H.; Tsao, C.-W.; Yen, W.-H.; Chang, W.-B.; Su, Y.-D.; Yeh, Y.-T.; et al. 5-Episinuleptolide acetate, a norcembranoidal diterpene from the formosan soft coral Sinularia sp., induces leukemia cell apoptosis through Hsp90 inhibition. Molecules 2013, 18, 2924–2933. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Su, J.-H.; Chen, Y.-C.; El-Shazly, M.; Du, Y.-C.; Su, C.-W.; Tsao, C.-W.; Liu, L.-L.; Chou, Y.; Chang, W.-B.; Su, Y.-D.; et al. Towards the Small and the Beautiful: A Small Dibromotyrosine Derivative from Pseudoceratina sp. Sponge Exhibits Potent Apoptotic Effect through Targeting IKK/NFκB Signaling Pathway. Mar. Drugs 2013, 11, 3168-3185. https://doi.org/10.3390/md11093168
Su J-H, Chen Y-C, El-Shazly M, Du Y-C, Su C-W, Tsao C-W, Liu L-L, Chou Y, Chang W-B, Su Y-D, et al. Towards the Small and the Beautiful: A Small Dibromotyrosine Derivative from Pseudoceratina sp. Sponge Exhibits Potent Apoptotic Effect through Targeting IKK/NFκB Signaling Pathway. Marine Drugs. 2013; 11(9):3168-3185. https://doi.org/10.3390/md11093168
Chicago/Turabian StyleSu, Jui-Hsin, Yu-Cheng Chen, Mohamed El-Shazly, Ying-Chi Du, Chiang-Wen Su, Chia-Wei Tsao, Li-Lian Liu, Yalan Chou, Wen-Been Chang, Yin-Di Su, and et al. 2013. "Towards the Small and the Beautiful: A Small Dibromotyrosine Derivative from Pseudoceratina sp. Sponge Exhibits Potent Apoptotic Effect through Targeting IKK/NFκB Signaling Pathway" Marine Drugs 11, no. 9: 3168-3185. https://doi.org/10.3390/md11093168




