Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review
Abstract
:1. Introduction
2. Source of Astaxanthin
| Sources | Astaxanthin (%) on the Dry Weight Basis | References |
|---|---|---|
| Chlorophyceae | ||
| Haematococcus pluvialis | 3.8 | [17,18] |
| Haematococcus pluvialis (K-0084) | 3.8 | [22] |
| Haematococcus pluvialis (Local isolation) | 3.6 | [23] |
| Haematococcus pluvialis (AQSE002) | 3.4 | [24] |
| Haematococcus pluvialis (K-0084) | 2.7 | [25] |
| Chlorococcum | 0.2 | [26,27] |
| Chlorella zofingiensis | 0.001 | [28] |
| Neochloris wimmeri | 0.6 | [29] |
| Ulvophyceae | ||
| Enteromorpha intestinalis | 0.02 | [30] |
| Ulva lactuca | 0.01 | [30] |
| Florideophyceae | ||
| Catenella repens | 0.02 | [30] |
| Alphaproteobacteria | ||
| Agrobacterium aurantiacum | 0.01 | [31] |
| Paracoccus carotinifaciens (NITE SD 00017) | 2.2 | [32] |
| Tremellomycetes | ||
| Xanthophyllomyces dendrorhous (JH) | 0.5 | [33] |
| Xanthophyllomyces dendrorhous (VKPM Y2476) | 0.5 | [34] |
| Labyrinthulomycetes | ||
| Thraustochytrium sp. CHN-3 (FERM P-18556) | 0.2 | [35] |
| Malacostraca | ||
| Pandalus borealis | 0.12 | [20] |
| Pandalus clarkia | 0.015 | [36] |

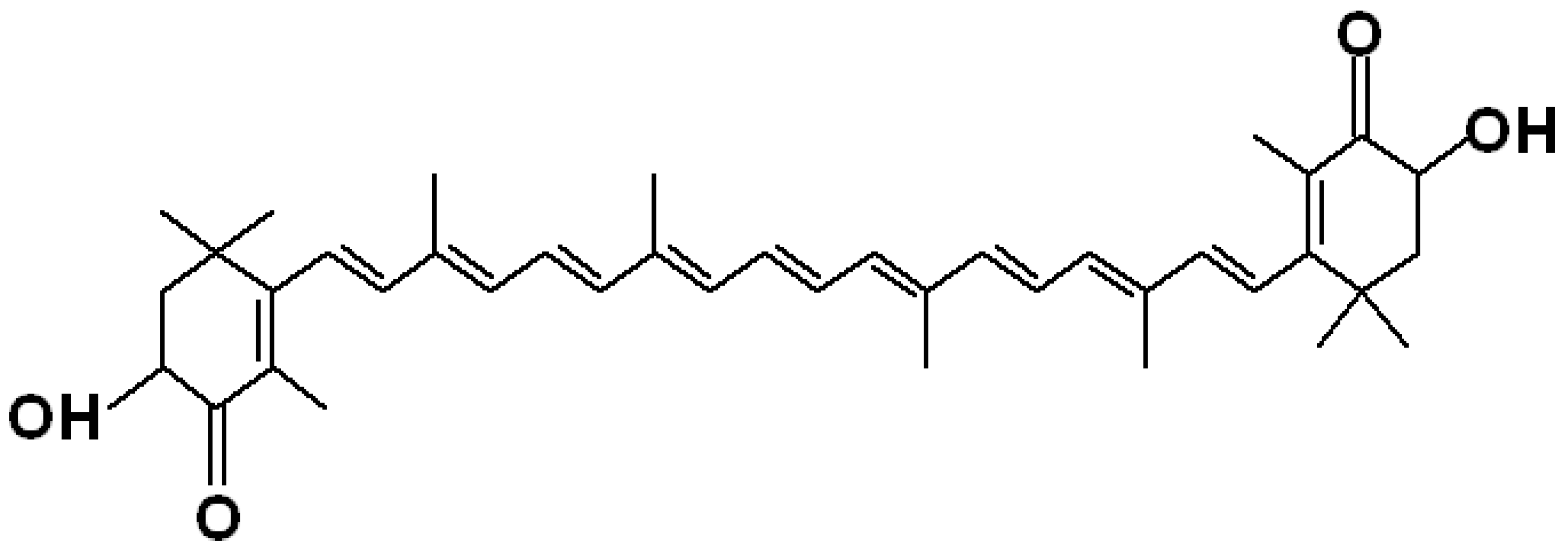
3. Structure of Astaxanthin
4. Extraction and Analysis of Astaxanthin
5. Storage and Stability of Astaxanthin
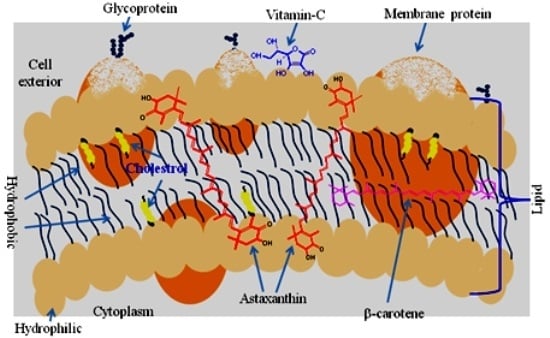
6. Biochemistry of Astaxanthin
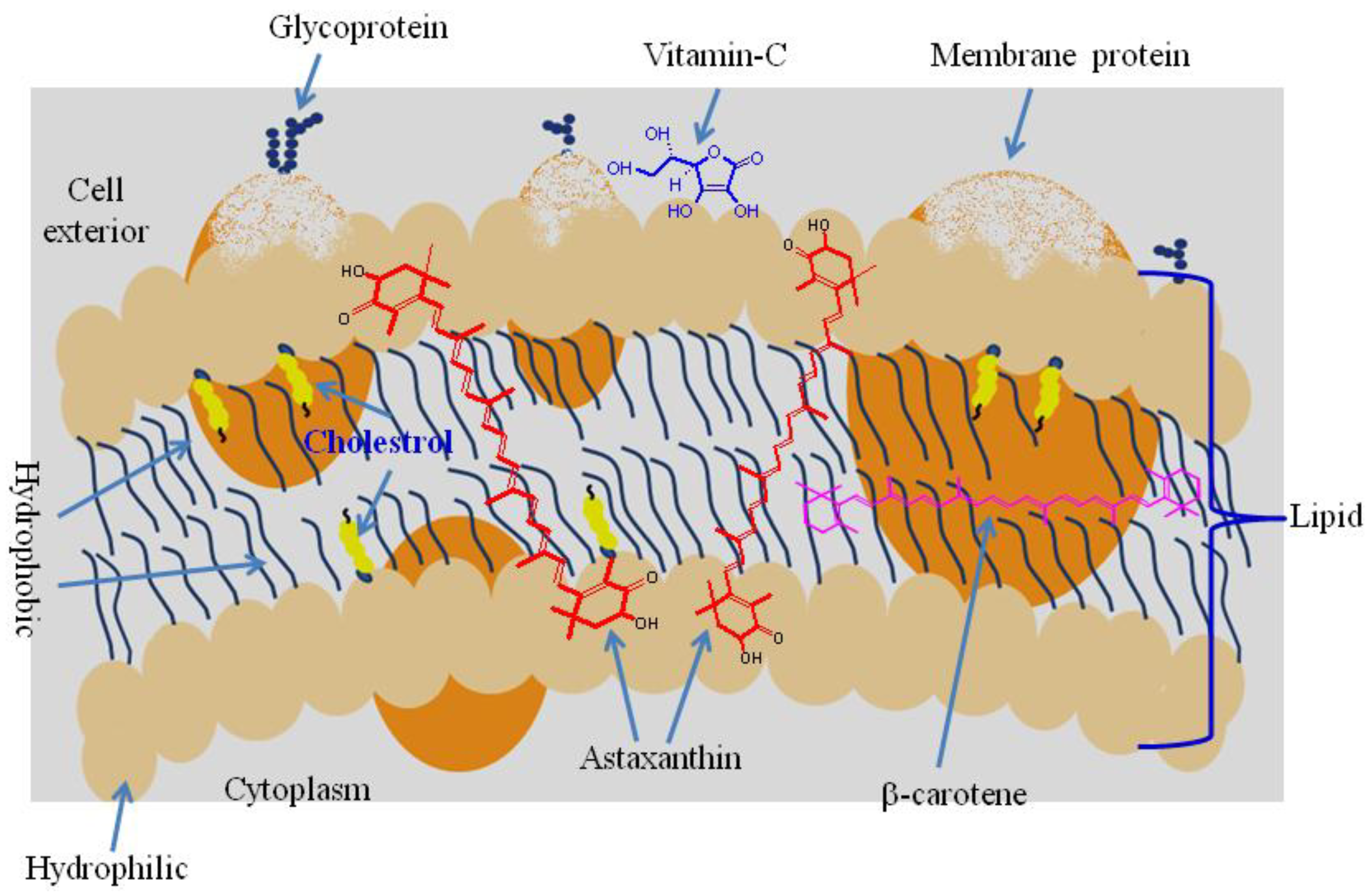
7. Bioavailability and Pharmacokinetics of Astaxanthin
7.1. Bioavailability
7.2. Pharmacokinetics
8. Biological Activities of Astaxanthin and Its Health Benefits
8.1. Antioxidant Effects
8.2. Anti-Lipid Peroxidation Activity
8.3. Anti-Inflammation
8.4. Anti-Diabetic Activity
8.5. Cardiovascular Disease Prevention
8.6. Anticancer Activity
8.7. Immuno-Modulation
| Biological Activities | References |
|---|---|
| Antioxidant activity | [14,15,17,115,116,117,118,119,120] |
| Protection from UV rays | [14] |
| Anti-skin cancer | [14,110,121] |
| Anti-inflammatory | [84,122,123,124,125] |
| Anti-gastric activity | [68,71] |
| Anti-hepatoprotective | [126] |
| Anti-diabetes | [90,127,128] |
| Cardiovascular prevention | [94,122,129,130] |
| Immune response | [72,114] |
| Neuroprotection | [131,132] |
9. Safety and Dose of Astaxanthin
| Duration of Experiment | Subjects in Humans | Dosage (mg/day) | Benefits of Astaxanthin | References |
|---|---|---|---|---|
| 2 weeks | Volunteers | 1.8, 3.6, 14.4 and 21.6 | Reduction of LDL oxidation | [21] |
| Single dose | Middle aged male volunteers | 100 | Astaxanthin take up by VLDL chylomicrons | [60] |
| 8 weeks | Healthy females | 0.2 and 8 | Decreased plasma 8-hydoxy-2′-deoxyguanosine and lowered in CRP levels | [72] |
| 8 weeks | Healthy adults | 6 | Assessed by blood pressure | [140] |
| 10 days | Healthy males | 6 | Improved blood rheology | [141] |
| 12 weeks | Healthy non-smoking finnish males | 8 | Decreased oxidation of fatty acids | [142] |
| 12 months | Age related macular degeneration | 4 | Improved central retinal dysfunction in age related macular degeneration | [143] |
| 12 weeks | Middle aged/elderly | 12 | Improved Cog health battery scores | [144] |
| 12 weeks | Middle aged/elderly | 6 | Improved groton maze learning test scores | [144] |
| 8 or 6 weeks | Healthy female or male | 6 | Improved skin winkle, corneocyte layer, epidermis and dermis | [145] |
| 2 weeks | Disease (bilateral cataract) | 6 | Improved superoxide scavenging activity and lowered hydroperoxides in the human aqueous humor | [146] |
10. Commercial Applications of Astaxanthin
| Brand Name | Dosage form | Ingredients | Company Name | Purpose |
|---|---|---|---|---|
| Physician Formulas | Soft gel/Tablets | 2 mg/4 mg-AX | Physician formulas vitamin company | Antioxidant |
| Eyesight Rx | Tablet | AX, vitamin-C, plant extracts | Physician formulas Vitamin company | Vision function |
| KriaXanthin | Soft gel | 1.5 mg-AX, EPA, DHA | Physician formulas vitamin company | Antioxidant |
| Astaxanthin Ultra | Soft gel | 4 mg-AX | AOR | Cardiovascular health/gastrointestinal |
| Astaxanthin Gold™ | Soft gel | 4 mg-AX | Nutrigold | Eye/joint/skin/immune health |
| Best Astaxanthin | Soft gel | 6 mg-AX, CX | Bioastin | Cell membrane/blood flow |
| Dr.Mercola | Capsules | 4 mg AX, 325 mg Omega-3 ALA | Dr. Mercola premium supplements | Aging/muscle |
| Solgar | Soft gel | 5 mg-AX | Solgar global manufacture | Healthy skin |
| Astaxanthin | Cream | AX, herbal extracts | True botanica | Face moisturizing |
| astavita ex | Capsules | 8 mg AX, T3 | Fuji Chemical Industry | Agingcare |
| astavita SPORT | Capsules | 9 mg AX, T3 and zinc | Fuji Chemical Industry | Sports nutrition |
| AstaREAL | Oil, powder, water soluble, biomass | AX, AX-esters | Fuji Chemical Industry | Soft gel, tablet, beverages, animal feed, capsules |
| AstaTROL | Oil | AX | Fuji Chemical Industry | Cosmetics |
| AstaFX | Capsules | AX | Purity and products evidence based nutritional supplements | Skin/cardiovascular function |
| Pure Encapsulations | Capsules | AX | Synergistic nutrition | Antioxidant |
| Zanthin Xp-3 | Soft gel capsules | 2 mg, 4 mg-AX | Valensa | Human body |
| Micro Algae Super Food | Soft gel | 4 mg AX | Anumed intel biomed company | heart/eye/joint |
| Patent No. | Title | Purpose | References |
|---|---|---|---|
| US20060217445 | Natural astaxanthin extract reduces DNA oxidation | Reduce endogenous oxidative damage | [147] |
| US20070293568 | Neurocyte protective agent | Neuroprotection | [148] |
| US20080234521 | Crystal forms of astaxanthin | Nutritional dosage | [149] |
| US20080293679 | Use of carotenoids and carotenoid derivatives analogs for reduction/ inhibition of certain negative effects of COX inhibitors | Inhibit of lipid peroxidation | [150] |
| US20090047304 | Composition for body fat reduction | Inhibits body fat | [151] |
| US20090069417 | Carotenoid oxidation products as chemopreventive and chemotherapeutic agents | Cancer prevention | [152] |
| US20090136469 | Formulation for oral administration with beneficial effects on the cardiovascular system | Cardiovascular protection | [153] |
| US20090142431 | Algal and algal extract dietary supplement composition | Dietary supplement | [154] |
| US20090297492 | Method for improving cognitive performance | Improving brain function | [155] |
| US20100158984 | Encapsulates | Capsules | [156] |
| US20100204523 | Method of preventing discoloration of carotenoid pigment and container used therefor | Prevention of discoloration | [157] |
| US20100267838 | Pulverulent carotenoid preparation for colouring drinks | Drinks | [158] |
| US20100291053 | Inflammatory disease treatment | Preventing inflammatory disease | [159] |
| US20120004297 | Agent for alleviating vascular failure | Preventing vascular failure | [160] |
| US20120114823 | Feed additive for improved pigment retention | Fish feed | [161] |
| US20120238522 | Carotenoid containing compositions and methods | Preventing bacterial infections | [162] |
| US20120253078 | Agent for improving carcass performance in finishing hogs | Food supplements | [163] |
| US20130004582 | Composition and method to alleviate joint pain | Reduced joint pain and symptoms of osteoarthritis | [164] |
| US20130108764 | Baked food produced from astaxanthin containing dough | Astaxanthin used in baked food | [165] |
11. Conclusion
Acknowledgments
Conflicts of Interest
References
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58D–68D. [Google Scholar]
- Roche, F. Astaxanthin: Human food safety summary. In Astaxanthin As a Pigmenter in Salmon Feed, Color Additive Petition 7C02 1 1, United States Food and Drug Administration; Hoffman-La Roche Ltd.: Basel, Switzerland, 1987; p. 43. [Google Scholar]
- Sarada, R.; Tripathi, U.; Ravishankar, G.A. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Ranga Rao, A. Production of astaxanthin from cultured green alga Haematococcus pluvialis and its biological activities. Ph.D. Thesis, University of Mysore, Mysore, India, 15 May 2011. [Google Scholar]
- Sarada, R.; Ranga Rao, A.; Sandesh, B.K.; Dayananda, C.; Anila, N.; Chauhan, V.S.; Ravishankar, G.A. Influence of different culture conditions on yield of biomass and value added products in microalgae. Dyn. Biochem. Proc. Biotechnol. Mol. Biol. 2012, 6, 77–85. [Google Scholar]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, B.; Lee, J.Y. Astaxanthin structure, metabolism, and health benefits. J. Hum. Nutr. Food Sci. 2013, 1, 1003:1–1003:11. [Google Scholar]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Yamashita, E. Astaxanthin as a medical food. Funct. Foods Health Dis. 2013, 3, 254–258. [Google Scholar]
- Dhankhar, J.; Kadian, S.S.; Sharma, A. Astaxanthin: A potential carotenoid. Int. J. Pharm. Sci. Res. 2012, 3, 1246–1259. [Google Scholar]
- Ranga Rao, A.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar]
- Ranga Rao, A.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. In vivo bioavailability and antioxidant activity of carotenoids from micro algal biomass—A repeated dose study. Food Res. Int. 2013, 54, 711–717. [Google Scholar] [CrossRef]
- Ranga Rao, A.; Harshvardhan Reddy, A.; Aradhya, S.M. Antibacterial properties of Spirulina platensis, Haematococcus pluvialis, Botryococcus braunii micro algal extracts. Curr. Trends Biotechnol. Pharm. 2010, 4, 809–819. [Google Scholar]
- Ranga Rao, A.; Raghunath Reddy, R.L.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. J. Agric. Food Chem. 2010, 58, 8553–8559. [Google Scholar] [CrossRef]
- Ranga Rao, A.; Sarada, R.; Baskaran, V.; Ravishankar, G.A. Identification of carotenoids from green alga Haematococcus pluvialis by HPLC and LC-MS (APCI) and their antioxidant properties. J. Microbiol. Biotechnol. 2009, 19, 1333–1341. [Google Scholar]
- Lorenz, R.T. A Technical Review of Haematococcus Algae; NatuRose™ Technical Bulletin #060; Cyanotech Corporation: Kailua-Kona, HI, USA, 1999; pp. 1–12. [Google Scholar]
- EFSA (European Food Safety Authority). Opinion of the scientific panel on additives and products or substances used in animal feed on the request from the European commission on the safety of use of colouring agents in animal human nutrition. EFSA J. 2005, 291, 1–40. [Google Scholar]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Thromb. 2000, 7, 216–222. [Google Scholar]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef]
- Torzillo, G.; Goksan, T.; Faraloni, C.; Kopecky, J.; Masojídek, J. Interplay between photochemical activities and pigment composition in an outdoor culture of Haematococcus pluvialis during the shift from the green to red stage. J. Appl. Phycol. 2003, 15, 127–136. [Google Scholar]
- Olaizola, M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000, 12, 499–506. [Google Scholar] [CrossRef]
- Wang, J.; Han, D.; Sommerfeld, M.R.; Lu, C.; Hu, Q. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J. Appl. Phycol. 2013, 25, 253–260. [Google Scholar] [CrossRef]
- Zhang, D.H.; Lee, Y.K. Enhanced accumulation of secondary carotenoids in a mutant of the green alga, Chlorococcum sp. J. Appl. Phycol. 1997, 9, 459–463. [Google Scholar] [CrossRef]
- Zhang, D.H.; Ng, M.L.; Phang, S.M. Composition and accumulation of secondary carotenoids in Chlorococcum sp. J. Appl. Phycol. 1997, 9, 147–155. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J. Growth associated biosynthesis of astaxanthin in heterotrophic Chlorella zofingiensis (Chlorophyta). World J. Microbiol. Biotechnol. 2008, 24, 1915–1922. [Google Scholar] [CrossRef]
- Orosa, M.; Torres, E.; Fidalgo, P.; Abalde, J. Production and analysis of secondary carotenoids in green algae. J. Appl. Phycol. 2000, 12, 553–556. [Google Scholar] [CrossRef]
- Banerjee, K.; Ghosh, R.; Homechaudhuri, S.; Mitra, A. Biochemical composition of marine macroalgae from gangetic delta at the apex of Bay of Bengal. Afr. J. Basic Appl. Sci. 2009, 1, 96–104. [Google Scholar]
- Yokoyama, A.; Adachi, K.; Shizuri, Y. New carotenoid glucosides, astaxanthin glucoside and adonimxanthin glucoside, isolated from the astaxanthin producing marine bacterium, Agrobacterium aurantiacum. J. Nat. Prod. 1995, 58, 1929–1933. [Google Scholar]
- EFSA (European Food Safety Authority). Safety and efficacy of panaferd-AX(red carotenoid rich bacterium Paracoccus carotinifaciens as feed additive for salmon and trout. EFSA J. 2007, 546, 1–30. [Google Scholar]
- Kim, J.H.; Kang, S.W.; Kim, S.W.; Chang, H.I. High-level production of astaxanthin by Xanthophyllomyces dendrorhous mutant JH1 using statistical experimental designs. Biosci. Biotechnol. Biochem. 2005, 69, 1743–1748. [Google Scholar] [CrossRef]
- De la Fuente, J.L.; Rodríguez-Sáiz, M.; Schleissner, C.; Díez, B.; Peiro, E.; Barredo, J.L. High-titer production of astaxanthin by the semi-industrial fermentation of Xanthophyllomyces dendrorhous. J. Biotechnol. 2010, 148, 144–146. [Google Scholar] [CrossRef]
- Yamaoka, Y. Microorganism and production of carotenoid compounds. U.S. Patent 7,374,908 B2, 20 May 2008. [Google Scholar]
- Meyers, S.P.; Bligh, D. Characterization of astaxanthin pigments from heat processed crawfish waste. J. Agric. Food Chem. 1981, 3, 505–508. [Google Scholar] [CrossRef]
- Foss, P.; Renstrøm, B.; Liaaen-Jensen, S. Natural occurrence of enantiomeric and meso astaxanthin. 7-crustaceans including zooplankton. Comp. Biochem. Physiol. B 1987, 86B, 313–314. [Google Scholar]
- Sarada, R.; Vidhyavathi, R.; Usha, D.; Ravishankar, G.A. An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis. J. Agric. Food Chem. 2006, 54, 7585–7588. [Google Scholar]
- Kobayashi, M.; Kurimura, Y.; Sakamoto, Y.; Tsuji, Y. Selective extraction of astaxanthin and chlorophyll from the green alga Haematococcus pluvialis. Biotechnol. Tech. 1997, 11, 657–660. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: Effects on astaxanthin recovery and implications for bio-availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Kang, C.D.; Sim, S.J. Direct extraction of astaxanthin from Haematococcus culture using vegetable oils. Biotechnol. Lett. 2008, 30, 441–444. [Google Scholar] [CrossRef]
- Ni, H.; Chen, Q.H.; He, G.Q.; Wu, G.B.; Yang, Y.F. Optimization of acidic extraction of astaxanthin from Phaffia rhodozyma. J. Zhejiang Univ. Sci. B 2008, 9, 51–59. [Google Scholar]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2010, 46, 64–70. [Google Scholar] [CrossRef]
- Storebakken, T.; Sørensen, M.; Bjerkeng, B.; Harris, J.; Monahan, P.; Hiu, S. Stability of astaxanthin from the red yeast, Xanthophyllomyces dendrorhous, during feed processing: Effects of enzymatic cell wall disruption and extrusion temperature. Aquaculture 2004, 231, 489–500. [Google Scholar] [CrossRef]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of astaxanthin from Haematococcus pluvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Nobre, B.; Marcelo, F.; Passos, R.; Beiro, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Wang, L.; Yang, B.; Yan, B.; Yao, X. Supercritical fluid extraction of astaxanthin from Haematococcus pluvialis and its antioxidant potential in sunflower oil. Innov. Food Sci. Emerg. Technol. 2012, 13, 120–127. [Google Scholar] [CrossRef]
- Ranga Rao, A.; Sarada, R.; Ravishankar, G.A. Stabilization of astaxanthin in edible oils and its use as an antioxidant. J. Sci. Food Agric. 2007, 87, 957–965. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P. Chemical stability of astaxanthin nanodispersions in orange juice and skimmed milk as model food systems. Food Chem. 2013, 139, 527–531. [Google Scholar] [CrossRef]
- Raposo, M.F.J.; Morais, A.M.M.B.; Morais, R.S.C. Effects of spray drying and storage on astaxanthin content of Haematococcus pluvialis biomass. World J. Microbiol. Biotechnol. 2012, 28, 1253–1257. [Google Scholar] [CrossRef]
- Villalobos-Castillejos, F.; Cerezal-Mezquita, P.; Hemandez-De Jesus, M.L.; Barragan-Huerta, B.E. Production and stability of water-dispersible astaxanthin oleoresin from Phaffia rhodozyma. Int. J. Food Sci. Technol. 2013, 48, 1243–1251. [Google Scholar] [CrossRef]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M.; Arguelles-Monal, W. Microencapsulation of astaxanthin in a chitosan matrix. Carbohydr. Polym. 2004, 56, 41–45. [Google Scholar] [CrossRef]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Rico, L.G.; Badolato, G.G.; Schubert, H. Production of O/W emulsions containing astaxanthin by repeated premix membrane emulsification. J. Food Sci. 2005, 70, E117–E123. [Google Scholar]
- Chen, X.; Chen, R.; Guo, Z.; Li, C.; Li, P. The preparation and stability of the inclusion complex of astaxanthin with β-cyclodextrin. Food Chem. 2007, 101, 1580–1584. [Google Scholar] [CrossRef]
- Barros, M.P.; Marin, D.P.; Bolin, A.P.; de Cássia Santos Macedo, R.; Campoio, T.R.; Fineto, C., Jr.; Guerra, B.A.; Polotow, T.G.; Vardaris, C.; Mattei, R.; et al. Combined astaxanthin and fish oil supplementation improves glutathione-based redox balance in rat plasma and neutrophils. Chem. Biol. Interact. 2012, 197, 58–67. [Google Scholar] [CrossRef]
- Otton, R.; Marin, D.P.; Bolin, A.P.; de Cássia Santos Macedo, R.; Campoio, T.R.; Fineto, C.J.; Guerra, B.A.; Leite, J.R.; Barros, M.P.; Mattei, R. Combined fish oil and astaxanthin supplementation modulates rat lymphocyte function. Eur. J. Nutr. 2012, 51, 707–718. [Google Scholar] [CrossRef]
- Page, G.I.; Davies, S.J. Astaxanthin and canthaxanthin do not induce liver or kidney xenobiotic-metabolizing enzymes in rainbow trout (Oncorhynchus mykiss Walbaum). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 133C, 443–451. [Google Scholar] [CrossRef]
- Osterlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z isomers in plasma lipoproteins of after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–492. [Google Scholar] [CrossRef]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of astaxanthin in Haematococcus algal extract: the effects of timing of diet and smoking habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef]
- Olson, J.A. Carotenoids: absorption, transport, and metabolism of carotenoids in humans. Pure Appl. Chem. 2004, 66, 1011–1016. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all trans isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Martin, H.D.; Jager, C.; Ruck, C.; Schmidt, M. Anti and pro-oxidant properties of carotenoids. J. Prakt. Chem. 1999, 341, 302–308. [Google Scholar] [CrossRef]
- Augusti, P.R.; Quatrin, A.; Somacal, S.; Conterato, G.M.; Sobieskim, R.; Ruviaro, A.R.; Maurer, L.H.; Duarte, M.M.; Roehrs, M.; Emanuelli, T. Astaxanthin prevents changes in the activities of thioredoxin reductase and paraoxonase in hypercholesterolemic rabbits. J. Clin. Biochem. Nutr. 2012, 51, 42–49. [Google Scholar] [CrossRef]
- Kamath, B.S.; Srikanta, B.M.; Dharmesh, S.M.; Sarada, R.; Ravishankar, G.A. Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur. J. Pharmacol. 2008, 590, 387–395. [Google Scholar] [CrossRef]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta 2001, 1512, 251–258. [Google Scholar]
- Liu, B.H.; Lee, Y.K. Effect of total secondary carotenoids extracts from Chlorococcum sp. on Helicobacter pylori infected BALB/c mice. Int. Immunopharmacol. 2003, 3, 979–986. [Google Scholar] [CrossRef]
- Bennedsen, M.; Wang, X.; Willen, R.; Wadstrom, T.; Andersen, L.P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 1999, 70, 185–189. [Google Scholar]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 1–10. [Google Scholar]
- Haines, D.D.; Varga, B.; Bak, I.; Juhasz, B.; Mahmoud, F.F; Kalantari, H.; Gesztelyi, R.; Lekli, I.; Czompa, A.; Tosaki, A. Summative interaction between astaxanthin, Ginkgo biloba extract (EGb761) and vitamin C in suppression of respiratory inflammation: A comparison with ibuprofen. Phytother. Res. 2011, 25, 128–136. [Google Scholar] [CrossRef]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ohgami, K.; Shiratori, K.; Jin, X.H.; Llieva, I.; Koyama, Y.; Yazawa, K.; Yoshidia, K.; Kase, S.; Ohno, S. Suppressive effects of astaxanthin against rat endotoxin induced uveitis by inhibiting the NF-kB signaling pathway. Exp. Eye Res. 2006, 82, 275–281. [Google Scholar] [CrossRef]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective effects of topical application of a poorly soluble antioxidant astaxanthin liposomal formulation on ultraviolet-induced skin damage. J. Pharm. Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef]
- Santos, S.D.; Cahú, T.B.; Firmino, G.O.; de Castro, C.C.; Carvalho, L.B.J.; Bezerra, R.S.; Filho, J.L. Shrimp waste extract and astaxanthin: Rat alveolar macrophage, oxidative stress and inflammation. J. Food Sci. 2012, 77, 141–146. [Google Scholar]
- Uchiyama, K.; Naito, Y.; Hasegawa, G.; Nakamura, N.; Takahashi, J.; Yoshikawa, T. Astaxanthin protects β-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002, 7, 290–293. [Google Scholar] [CrossRef]
- Otton, R.; Marin, D.P.; Bolin, A.P.; Santos, R.C.; Polotow, T.G.; Sampaio, S.C.; De Barros, M.P. Astaxanthin ameliorates the redox imbalance in lymphocytes of experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 306–315. [Google Scholar] [CrossRef]
- Nakano, M.; Onodera, A.; Saito, E.; Tanabe, M.; Yajima, K.; Takahashi, J.; Nguyen, V.C. Effect of astaxanthin in combination with α-tocopherol or ascorbic acid against oxidative damage in diabetic ODS rats. J. Nutr. Sci. Vitaminol. 2008, 54, 329–334. [Google Scholar] [CrossRef]
- Nishigaki, I.; Rajendran, P.; Venugopal, R.; Ekambaram, G.; Sakthisekaran, D.; Nishigaki, Y. Cytoprotective role of astaxanthin against glycated protein/iron chelate-induced toxicity in human umbilical vein endothelial cells. Phytother. Res. 2010, 24, 54–59. [Google Scholar] [CrossRef]
- Hussein, G.; Nakagawa, T.; Goto, H.; Shimada, Y.; Matsumoto, K.; Sankawa, U.; Watanabe, H. Astaxanthin ameliorates features of metabolic syndrome in SHR/NDmcr-cp. Life Sci. 2007, 80, 522–529. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Arunkumar, E.; Viswanathan, P.; Anuradha, C.V. Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed an obesity-promoting diet. Process Biochem. 2010, 45, 1406–1414. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Yogalakshmi, B.; Sreeja, S.; Anuradha, C.V. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-κB-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones 2013, in press. [Google Scholar]
- Naito, Y.; Uchiyama, K.; Aoi, W.; Hasegawa, G.; Nakamura, N.; Yoshida, N.; Maoka, T.; Takahashi, J.; Yoshikawa, T. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. BioFactors 2004, 20, 49–59. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.A.; Yokozawa, T. Protection against oxidative stress, inflammation, and apoptosis of high glucose- exposed proximal tubular epithelial cells by astaxanthin. J. Agric. Food Chem. 2009, 57, 8793–8797. [Google Scholar] [CrossRef]
- Manabe, E.; Handa, O.; Naito, Y.; Mizushima, K.; Akagiri, S.; Adachi, S.; Takagi, T.; Kokura, S.; Maoka, T.; Yoshikawa, T. Astaxanthin protects mesangial cells from hyperglycemia induced oxidative signaling. J. Cell Biochem. 2008, 103, 1925–1937. [Google Scholar] [CrossRef]
- Fassett, R.G.; Combes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef]
- Lauver, D.A.; Lockwood, S.F.; Lucchesi, B.R. Disodium disuccinate astaxanthin (Cardax) attenuates complement activation and reduces myocardial injury following ischemia/reperfusion. J. Pharmacol. Exp. Ther. 2005, 314, 686–692. [Google Scholar] [CrossRef]
- Gross, G.J.; Lockwood, S.F. Acute and chronic administration of disodium disuccinate astaxanthin (Cardax) produces marked cardioprotection in dog hearts. Mol. Cell. Biochem. 2005, 272, 221–227. [Google Scholar] [CrossRef]
- Gross, G.J.; Hazen, S.L.; Lockwood, S.F. Seven day oral supplementation with Cardax (disodium disuccinate astaxanthin) provides significant cardioprotection and reduces oxidative stress in rats. Mol. Cell. Biochem. 2006, 283, 23–30. [Google Scholar] [CrossRef]
- Monroy-Ruiz, J.; Sevilla, M.Á.; Carrón, R.; Montero, M.J. Astaxanthin-enriched-diet reduces blood pressure and improves cardiovascular parameters in spontaneously hypertensive rats. Pharmacol. Res. 2011, 63, 44–50. [Google Scholar] [CrossRef]
- Khan, S.K.; Malinski, T.; Mason, R.P.; Kubant, R.; Jacob, R.F.; Fujioka, K.; Denstaedt, S.J.; King, T.J.; Jackson, H.L.; Hieber, A.D.; et al. Novel astaxanthin prodrug (CDX-085) attenuates thrombosis in a mouse model. Thromb. Res. 2010, 126, 299–305. [Google Scholar] [CrossRef]
- Nakao, R.; Nelson, O.L.; Park, J.S.; Mathison, B.D.; Thompson, P.A.; Chew, B.P. Effect of astaxanthin supplementation on inflammation and cardiac function in BALB/c mice. Anticancer Res. 2010, 30, 2721–2725. [Google Scholar]
- Ryu, S.K.; King, T.J.; Fujioka, K.; Pattison, J.; Pashkow, F.J.; Tsimikas, S. Effect of an oral astaxanthin prodrug (CDX-085) on lipoprotein levels and progression of atherosclerosis in LDLR and ApoE mice. Atherosclerosis 2012, 222, 99–105. [Google Scholar] [CrossRef]
- Wolf, G. Retinoids and carotenoids as inhibitors of carcinogenesis and inducers of cell-cell communication. Nutr. Rev. 1992, 50, 270–274. [Google Scholar] [CrossRef]
- Hanusch, M.; Stahl, W.; Schulz, W.A.; Sies, H. Induction of gap junctional communication by 4-oxoretinoic acid generated from its precursor canthaxanthin. Arch. Biochem. Biophys. 1995, 317, 423–428. [Google Scholar] [CrossRef]
- Hix, L.M.; Lockwood, S.F.; Bertram, J.S. Upregulation of connexin 43 protein expression and increased gap junctional communication by water soluble disodium disuccinate astaxanthin derivatives. Cancer Lett. 2006, 211, 25–37. [Google Scholar]
- Daubrawa, F.; Sies, H.; Stahl, W. Astaxanthin diminishes gap junctional intercellular communication in primary human fibroblasts. J. Nutr. 2005, 135, 2507–2511. [Google Scholar]
- Zhang, L.X.; Cooney, R.V.; Bertram, J.S. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis 1991, 12, 2109–2114. [Google Scholar] [CrossRef]
- Zhang, L.X.; Cooney, R.V.; Bertram, J.S. Carotenoids up-regulate connexin-43 gene expression independent of their provitamin A or antioxidant properties. Cancer Res. 1992, 52, 5707–5712. [Google Scholar]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar]
- Chew, B.P.; Park, J.S.; Wong, M.W.; Wong, T.S. A comparison of the anticancer activities of dietary β-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999, 19, 1849–1853. [Google Scholar]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Mori, H.; Satoh, K.; Hara, A. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophyll’s, astaxanthin and canthaxanthin. Cancer Res. 1995, 55, 4059–4064. [Google Scholar]
- Tanaka, T.; Morishita, Y.; Suzui, M.; Kojima, T.; Okumura, A.; Mori, H. Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin. Carcinogenesis 1994, 15, 15–19. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumor activity of astaxanthin and its mode of action. Nutr. Cancer 2000, 36, 59–65. [Google Scholar] [CrossRef]
- Prabhu, P.N.; Ashokkumar, P.; Sudhandiran, G. Antioxidative and anti-proliferative effects of astaxanthin during the initiation stages of 1,2-dimethyl hydrazineinduced experimental colon carcinogenesis. Fund. Clin. Pharmacol. 2009, 23, 225–234. [Google Scholar] [CrossRef]
- Nakao, R.; Nelson, O.L.; Park, J.S.; Mathison, B.D.; Thompson, P.A.; Chew, B.P. Effect of dietary astaxanthin at different stages of mammary tumor initiation in BALB/c mice. Anticancer Res. 2010, 30, 2171–2175. [Google Scholar]
- Maoka, T.; Tokuda, H.; Suzuki, N.; Kato, H.; Etoh, H. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenesis activities of nitroastaxanthin and nitrolutein, the reaction products of astaxanthin and lutein with peroxynitrite. Mar. Drugs 2012, 10, 1391–1399. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Hill, R.; Tomita, Y.; Good, R. Studies of immunomodulating actions of carotenoids. I. Effects of β-carotene and astaxanthin on murine lymphocyte functions and cell surface marker expression in in vitro culture system. Nutr. Cancer 1991, 16, 93–105. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Zhang, L.; Gross, M.; Tomita, Y. Immunomodulating actions of carotenoids: Enhancement of in vivo and in vitro antibody production to T-dependent antigens. Nutr. Cancer 1994, 21, 47–58. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Gross, M. Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: astaxanthin, a carotenoid without vitamin A activity, enhances in vitro immunoglobulin production in response to a T-dependent stimulant and antigen. Nutr. Cancer 1995, 23, 171–183. [Google Scholar] [CrossRef]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Chew, B.P. Astaxanthin stimulates cell-mediated and humoral immune responses in cats. Vet. Immunol. Immunopathol. 2011, 144, 455–461. [Google Scholar] [CrossRef]
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br. J. Nutr. 2011, 105, 220–227. [Google Scholar] [CrossRef]
- Sila, A.; Ayed-Ajmi, Y.; Sayari, N.; Nasri, M.; Martinez-Alvarez, O.; Bougatef, A. Antioxidant and anti-proliferative activities of astaxanthin extracted from the shell waste of deep-water pink shrimp (Parapenaeus longirostris). Nat. Prod. J. 2013, 3, 82–89. [Google Scholar]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.K.; Kim, J.T.; Oh, J.M.; Shin, W.G. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Carpentero Burdeos, G.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef]
- Yang, Y.; Seo, J.M.; Nguyen, A.; Pham, T.X.; Park, H.J.; Park, Y.; Kim, B.; Bruno, R.S.; Lee, J. Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J. Nutr. 2011, 141, 1611–1617. [Google Scholar] [CrossRef]
- Ishiki, M.; Nishida, Y.; Ishibashi, H.; Wada, T.; Fujisaka, S.; Takikawa, A.; Urakaze, M.; Sasaoka, T.; Usui, I.; Tobe, K. Impact of divergent effects of astaxanthin on insulin signaling in l6 cells. Endocrinology 2013, 154, 2600–2612. [Google Scholar] [CrossRef]
- Huangfu, J.; Liu, J.; Sun, Z.; Wang, M.; Jiang, Y.; Chen, Z.Y.; Chen, F. Anti-ageing effects of astaxanthin-rich alga Haematococcus pluvialis on fruit flies under oxidative stress. J. Agric. Food Chem. 2013, 6, 7800–7804. [Google Scholar]
- Chew, W.; Mathison, B.D.; Kimble, L.L.; Mixter, P.F.; Chew, B.P. Astaxanthin decreases inflammatory biomarkers associated with cardiovascular disease in human umbilical vein endothelial cells. Am. J. Adv. Food Sci. Technol. 2013, 1, 1–17. [Google Scholar]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Zhang, J.; Reinhart, G.A.; Chew, B.P. Astaxanthin modulates age-associated mitochondrial dysfunction in healthy dogs. J. Animal Sci. 2013, 91, 268–275. [Google Scholar] [CrossRef]
- Gal, A.F.; Andrei, S.; Cernea, C.; Taulescu, M.; Catoi, C. Effects of astaxanthin supplementation on chemically induced tumorigenesis in Wistar rats. Acta Vet. Scand. 2012, 54, 1–6. [Google Scholar] [CrossRef]
- Wibrand, K.; Berge, K.; Messaoudi, M.; Duffaud, A.; Panja, D.; Bramham, C.R.; Burri, L. Enhanced cognitive function and antidepressant-like effects after krill oil supplementation in rats. Lipids Health Dis. 2013, 12, 1–13. [Google Scholar] [CrossRef]
- Turkez, H.; Geyikoglu, F.; Yousef, M.I. Beneficial effect of astaxanthin on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced liver injury in rats. Toxicol. Ind. Health 2012, 29, 591–599. [Google Scholar] [CrossRef]
- Chan, K.C.; Pen, P.J.; Yin, M.C. Anti-coagulatory and anti-inflammatory effects of astaxanthin in diabetic rats. J. Food Sci. 2012, 77, H76–H80. [Google Scholar] [CrossRef]
- Dong, L.Y.; Jin, J.; Lu, G.; Kang, X.L. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar. Drugs 2013, 11, 960–974. [Google Scholar] [CrossRef]
- Iizuka, M.; Ayaori, M.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Sasaki, M.; Komatsu, T.; Yogo, M.; et al. Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J. Nutr. Sci. Vitaminol. (Tokyo) 2012, 58, 96–104. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Chang, C.H.; Chen, C.Y.; Chiou, J.Y.; Peng, R.Y.; Peng, C.H. Astaxanthin secured apoptic death of PC12 cells induced by β-amyloid peptide 25–35: Its molecular action targets. J. Med. Food 2010, 13, 548–556. [Google Scholar] [CrossRef]
- Lu, Y.P.; Liu, S.Y.; Sun, H.; Wu, X.M.; Li, J.J.; Zhu, L. Neuroprotective effect of astaxanthin on H2O2-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010, 1360, 40–48. [Google Scholar]
- Stewart, J.S.; Lignell, A.; Pettersson, A.; Elfving, E.; Soni, M.G. Safety assessment of astaxanthin rich microalgae biomass: acute and subchronic toxicity studies in rats. Food Chem. Toxicol. 2008, 46, 3030–3036. [Google Scholar] [CrossRef]
- Hussein, G.; Nakamura, M.; Zhao, Q.; Iguchi, T.; Goto, H.; Sankawa, U.; Watanabe, H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol. Pharm. Bull. 2005, 28, 47–52. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Song, G.G.; Park, J.J.; Chang, H.I. Protective effect of astaxanthin on naproxen-induced gastric antral ulceration in rats. Eur. J. Pharmacol. 2005, 514, 53–59. [Google Scholar] [CrossRef]
- Petri, D.; Lundebye, A.K. Tissue distribution of astaxanthin in rats following exposure to graded levels in the feed. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 202–209. [Google Scholar] [CrossRef]
- Murata, K.; Oyagi, A.; Takahira, D.; Tsuruma, K.; Shimazawa, M.; Ishibashi, T.; Hara, H. Protective effects of astaxanthin from paracoccus carotinifaciens on murine gastric ulcer models. Phytother. Res. 2012, 26, 1126–1132. [Google Scholar] [CrossRef]
- Odeberg, M.J.; Lignell, A.; Pettersson, A.; Hoglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Serebruany, V.; Malinin, A.; Goodin, T.; Pashkow, F. The in vitro effects of xancor, a synthetic astaxanthine derivative, on hemostatic biomarkers in aspirin-naive and aspirin-treated subjects with multiple risk factors for vascular disease. Am. J. Ther. 2010, 17, 125–132. [Google Scholar] [CrossRef]
- Spiller, G.A.; Dewell, A. Safety of an astaxanthin rich Haemaotoccu pluvialis algal extract: A randomized clinical trial. J. Med. Food 2003, 6, 51–56. [Google Scholar] [CrossRef]
- Miyawaki, H.; Takahashi, J.; Tsukahara, H.; Takehara, I. Effects of astaxanthin on human blood rheology. J. Clin. Biochem. Nutr. 2008, 43, 69–74. [Google Scholar] [CrossRef]
- Karppi, J.; Rissanen, T.H.; Nyyssonen, K.; Kaikkonen, J.; Olsson, A.G.; Voutilainen, S.; Salonen, J.T. Effects of astaxanthin supplementation on lipid peroxidation. Int. J. Vitam. Nutr. Res. 2007, 77, 3–11. [Google Scholar] [CrossRef]
- Parisi, V.; Tedeschi, M.; Gallinaro, G.; Varano, M.; Saviano, S.; Piermarocchi, S. Carotenoids and antioxidants in age-related maculopathy italian study: multifocal electroretinogram modifications after one year. Ophthalmology 2008, 115, 324–333. [Google Scholar] [CrossRef]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of astaxanthin rich Haematococcus pluvialis extact on cognitive function: Arandomised double blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar]
- Hashimoto, H.; Arai, K.; Hayashi, S.; Okamoto, H.; Takahashi, J.; Chikuda, M.; Obara, Y. Effects of astaxanthin on antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 2013, 53, 1–7. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Natural astaxanthin extract reduces DNA oxidation. Patent US20060217445, 28 September 2006. [Google Scholar]
- Tsuji, S.; Shirasawa, T.; Shimizu, T. Neurocyte protective agent. Patent US20070293568, 23 December 2007. [Google Scholar]
- Leigh, S.; Steven Leight, M.L.; Hogevest, P.V. Crystal forms of astaxanthin. Patent US20080234521, 25 September 2007. [Google Scholar]
- Lockwood, S.F.; Preston, M. Use of carotenoids and or carotenoid derivatives analogs for reduction/inhibition of certain negative effects of COX inhibitors. Patent US20080293679, 27 November 2008. [Google Scholar]
- Takahashi, J.; Yamashita, E.; Fukamauchi, M.; Tanka, I. Composition for body fat reduction. Patent US20090047304, 8 June 2009. [Google Scholar]
- Sharoni, Y.; Levy, J.; Sela, Y.; Nir, Z. Carotenoid oxidation products as chemo preventive and chemotherapeutic agents. Patent US20090069417, 12 March 2009. [Google Scholar]
- Senin, P.; Setnikar, I.; Rovati, A. Formulation for oral administration with beneficial effects on the cardiovascular system. U.S. Patent 20090136469, 28 May 2009. [Google Scholar]
- David, A.E.; Melchior, R. Algal and algal extract dietary supplement composition. Patent US20090142431, 4 June 2009. [Google Scholar]
- Satoh, A.; Tsuji, S. Method for improving cognitive performance. Patent US20090297492, 3 December 2009. [Google Scholar]
- Qvyjt, F. Encapsulates. Patent US20100158984, 24 June 2010. [Google Scholar]
- Tominaga, K.; Karato, M.; Hongo, N.; Yamashita, E. Method of preventing discoloration of carotenoid pigment and container used therefor. Patent US20100204523, 12 August 2010. [Google Scholar]
- Kopsel, C. Pulverulent carotenoid preparation for colouring drinks. Patent US20100267838, 21 October 2010. [Google Scholar]
- Clayton, D.; Rutter, R. Inflammatory disease treatment. Patent US20100291053, 18 November 2010. [Google Scholar]
- Higashi, N.; Takahashi, J. Agent for alleviating vascular failure. Patent US20120004297, 5 Jannuary 2012. [Google Scholar]
- Koppe, W.M.; Moeller, N.P.; Baardsen, G.K.L. Feed additive for improved pigment retention. Patent US20120114823, 10 May 2012. [Google Scholar]
- Jouni, Z.; Makhoul, Z. Carotenoid containing compositions and methods. Patent US20120238522, 20 September 2012. [Google Scholar]
- Monahan, P.; Hiu, S. Agent for improving carcass performance in finishing hogs. Patent US20120253078, 4 October 2012. [Google Scholar]
- Minatelli, J.A.; Thomas, S.; Rajendran, L.; Moerck, E. Composition and method to alleviate joint pain. Patent US20130004582, 3 January 2013. [Google Scholar]
- Ooi, Y.; Kitamura, A.; Yamashita, E. Baked food produced from astaxanthin containing dough. Patent US 20130108764, 2 May 2013. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128-152. https://doi.org/10.3390/md12010128
Ambati RR, Phang S-M, Ravi S, Aswathanarayana RG. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Marine Drugs. 2014; 12(1):128-152. https://doi.org/10.3390/md12010128
Chicago/Turabian StyleAmbati, Ranga Rao, Siew-Moi Phang, Sarada Ravi, and Ravishankar Gokare Aswathanarayana. 2014. "Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review" Marine Drugs 12, no. 1: 128-152. https://doi.org/10.3390/md12010128