Discovery of Novel Diterpenoids from Sinularia arborea
Abstract
:1. Introduction

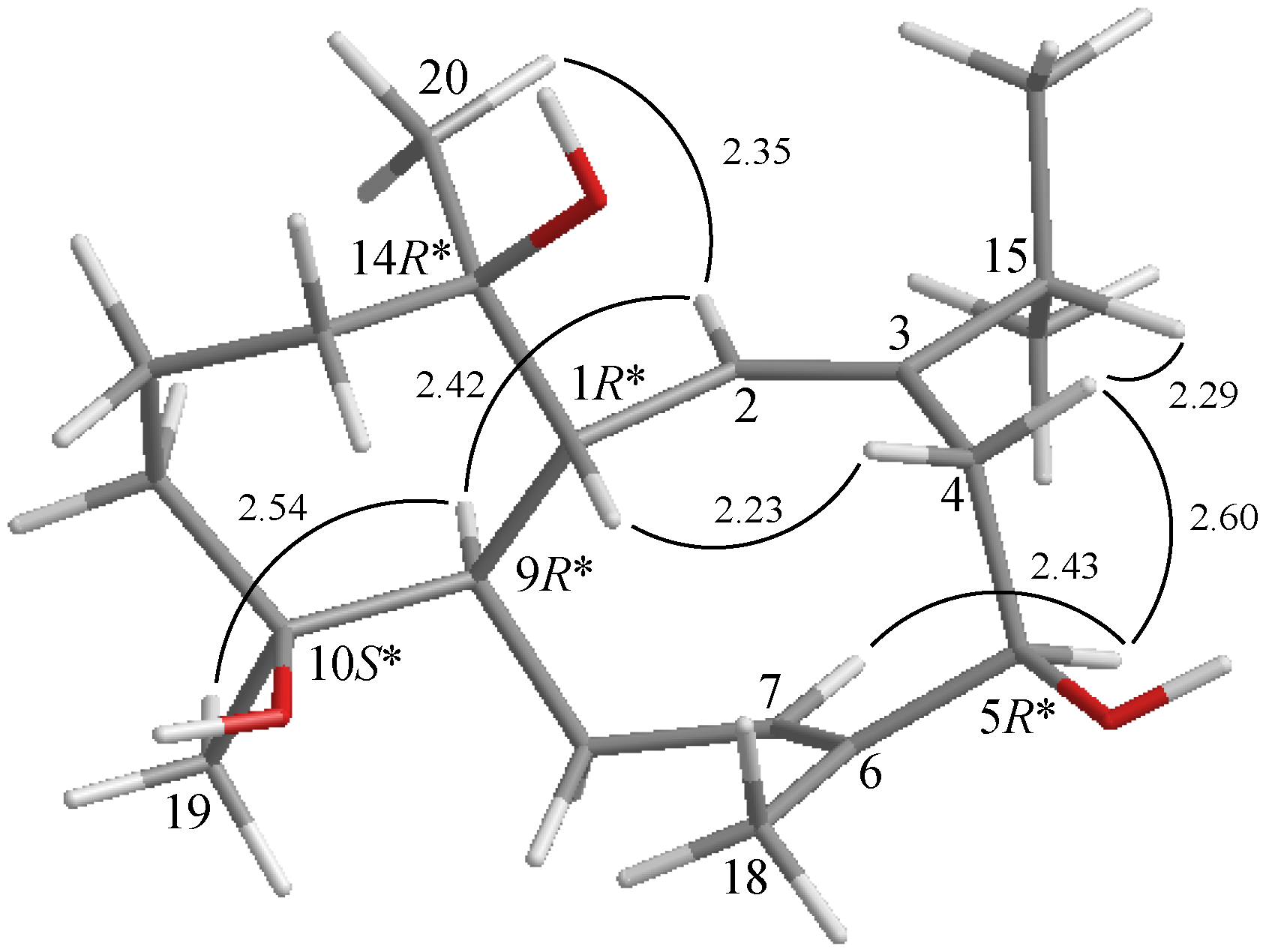
2. Results and Discussion
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC | |
|---|---|---|---|---|---|
| 1 | 2.45 dd (11.2, 9.6) | 49.9 | CH | H-2, H-9 | C-2, -3, -9, -10, -14 |
| 2 | 5.20 d (9.6) | 125.5 | CH | H-1 | C-1, -4, -9, -15 |
| 3 | 146.7 | C | |||
| 4 | 2.12 dd (12.8, 2.8) | 38.3 | CH2 | H-5 | C-2, -3, -5, -6, -15 |
| 2.94 dd (12.8, 11.2) | |||||
| 5 | 4.03 ddd (11.2, 2.8, 2.8) | 76.0 | CH | H2-4, OH-5 | n.o. a |
| 6 | 137.4 | C | |||
| 7 | 5.58 dd (6.4, 6.4) | 127.7 | CH | H2-8 | C-5, -18 |
| 8 | 2.16 m; 2.27 m | 22.4 | CH2 | H-7, H-9 | C-7, -10 |
| 9 | 1.97 m | 56.6 | CH | H-1, H2-8 | n.o. |
| 10 | 74.7 | C | |||
| 11 | 1.62 m; 1.98 m | 33.9 | CH2 | H2-12 | C-9, -10 |
| 12 | 1.34 m; 1.66 m | 23.4 | CH2 | H2-11, H2-13 | n.o. |
| 13 | 1.68–1.75 m | 39.5 | CH2 | H2-12 | C-1, -12, -14 |
| 14 | 81.5 | C | |||
| 15 | 2.30 m | 34.6 | CH | H3-16, H3-17 | C-2, -3, -4, -16, -17 |
| 16 | 1.09 d (6.8) | 24.1 | CH3 | H-15 | C-15, -17 |
| 17 | 1.09 d (6.8) | 24.2 | CH3 | H-15 | C-15, -17 |
| 18 | 1.78 s | 12.7 | CH3 | C-5, -6, -7 | |
| 19 | 1.16 s | 31.3 | CH3 | C-9, -10, -11 | |
| 20 | 1.09 s | 22.2 | CH3 | C-1, -13, -14 | |
| OH-5 | 1.53 d (2.8) | H-5 | n.o. | ||
| OH-10 | 2.08 s | C-9, -10, -19 | |||
| OH-14 | 2.18 s | C-13, -14 | |||
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC | |
|---|---|---|---|---|---|
| 1 | 2.54 dd (11.2, 9.6) | 50.2 | CH | H-2, H-9 | C-2, -3, -9, -10, -14, -20 |
| 2 | 5.19 dd (9.6) | 124.0 | CH | H-1 | C-1, -4, -9, -15 |
| 3 | 149.0 | C | |||
| 4 | 1.99 m | 29.5 | CH2 | H2-5 | C-2, -3, -5, -6, -15 |
| 2.72 ddd (13.2, 13.2, 2.8) | |||||
| 5 | 1.88 m; 2.22 m | 35.9 | CH2 | H2-4 | C-3, -4, -6, -7, -18 |
| 6 | 134.8 | C | |||
| 7 | 5.32 dd (5.6, 5.6) | 127.7 | CH | H2-8 | n.o. a |
| 8 | 2.08 m; 2.30 m | 22.8 | CH2 | H-7, H-9 | C-7, -10 |
| 9 | 1.97 m | 56.6 | CH | H-1, H2-8 | n.o. |
| 10 | 74.7 | C | |||
| 11 | 1.59 m; 1.88 m | 34.1 | CH2 | H2-12 | C-9, -12, -19 |
| 12 | 1.35 m; 1.66 m | 23.3 | CH2 | H2-11, H2-13 | C-10 |
| 13 | 1.68–1.77 m | 39.2 | CH2 | H2-12 | C-1, -14, -20 |
| 14 | 81.5 | C | |||
| 15 | 2.27 m | 33.2 | CH | H3-16, H3-17 | C-2, -3, -16, -17 |
| 16 | 1.06 d (6.8) | 24.1 | CH3 | H-15 | C-3, -15, -17 |
| 17 | 1.09 d (6.8) | 24.4 | CH3 | H-15 | C-3, -15, -16 |
| 18 | 1.70 s | 18.2 | CH3 | C-5, -6, -7 | |
| 19 | 1.51 s | 31.6 | CH3 | C-9, -10, -11 | |
| 20 | 1.08 s | 22.1 | CH3 | C-1, -13, -14 | |
| Compound | Superoxide anion | Elastase release |
|---|---|---|
| Inh% | Inh% | |
| 1 | 7.37 ± 1.98 * | 11.71 ± 1.35 *** |
| 2 | 23.94 ± 6.35 * | 6.54 ± 3.54 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
 −47 (c 0.04, CHCl3); IR (neat) υmax 3435 cm–1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1; ESIMS: m/z 345 [M + Na]+; HRESIMS: m/z 345.2404 (calcd. for C20H34O3Na, 345.2406).
−47 (c 0.04, CHCl3); IR (neat) υmax 3435 cm–1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1; ESIMS: m/z 345 [M + Na]+; HRESIMS: m/z 345.2404 (calcd. for C20H34O3Na, 345.2406). −8 (c 0.05, CHCl3); IR (neat) υmax 3447 cm–1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2; ESIMS: m/z 327 [M + Na]+; HRESIMS: m/z 329.2453 (calcd. for C20H34O2Na, 329.2456).
−8 (c 0.05, CHCl3); IR (neat) υmax 3447 cm–1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2; ESIMS: m/z 327 [M + Na]+; HRESIMS: m/z 329.2453 (calcd. for C20H34O2Na, 329.2456).3.4. Generation of Superoxide Anions and Release of Elastase by Human Neutrophils
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Chen, W.-T.; Li, Y.; Guo, Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–237. [Google Scholar]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef]
- Verseveldt, J. Octocorallia from North-Western Madagascar (Part II). Zool. Verh. 1971, 117, 1–73. [Google Scholar]
- Chen, K.-H.; Dai, C.-F.; Lu, M.-C.; Li, J.-J.; Chen, J.-J.; Chang, Y.-C.; Su, Y.-D.; Wang, W.-H.; Sung, P.-J. Secondary metabolites from the soft coral Sinularia arborea. Mar. Drugs 2013, 11, 3372–3380. [Google Scholar] [CrossRef]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- In the in vitro anti-inflammatory bioassay, the inhibitory effects on the generation of superoxide anion and the release of elastase by activated neutrophils were used as indicators. For significant activity of pure compounds, an inhibition rate ≥ 50% is required (inhibition rate ≤ 10%, not active; 20% ≥ inhibition rate ≥ 10%, weakly anti-inflammatory; 50% ≥ inhibition rate ≥ 20%, modestly anti-inflammatory).
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluoro-benzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Wang, C.-C.; Kuo, Y.-H.; Huang, H.-C.; Wu, Y.-C.; Kuo, L.-M.; Wu, Y.-H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Su, Y.-C.; Chang, H.-L.; Leu, Y.-L.; Chung, P.-J.; Kuo, L.-M.; Chang, Y.-J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009, 50, 1395–1408. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Li, G.-L.; Lan, Y.-H.; Chia, Y.-C.; Hsieh, P.-W.; Wu, Y.-H.; Wu, Y.-C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med. 2009, 46, 520–528. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Leu, Y.-L.; Kao, S.-H.; Tang, M.-C.; Chang, H.-L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radic. Biol. Med. 2006, 41, 1433–1441. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Yeh, S.-H.; Leu, Y.-L.; Chern, C.-Y.; Hsu, H.-C. Inhibition of superoxide anion and elastase release in human neutrophils by 3′-isopropoxychalcone via a cAMP-dependent pathway. Br. J. Pharmacol. 2006, 148, 78–87. [Google Scholar]
- Hwang, T.-L.; Hung, H.-W.; Kao, S.-H.; Teng, C.-M.; Wu, C.-C.; Cheng, S.J.-S. Soluble guanylyl cyclase activator YC-1 inhibits human neutrophil functions through a cGMP-independent but cAMP-dependent pathway. Mol. Pharmacol. 2003, 64, 1419–1427. [Google Scholar] [CrossRef]
- Wahlberg, I.; Eklund, A.-M. Cyclized cembranoids of natural occurrence. Prog. Chem. Org. Nat. Prod. 1992, 60, 1–141. [Google Scholar]
- Berrue, F.; Kerr, R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
Supplementary Files
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, K.-H.; Dai, C.-F.; Hwang, T.-L.; Chen, C.-Y.; Li, J.-J.; Chen, J.-J.; Wu, Y.-C.; Sheu, J.-H.; Wang, W.-H.; Sung, P.-J. Discovery of Novel Diterpenoids from Sinularia arborea. Mar. Drugs 2014, 12, 385-393. https://doi.org/10.3390/md12010385
Chen K-H, Dai C-F, Hwang T-L, Chen C-Y, Li J-J, Chen J-J, Wu Y-C, Sheu J-H, Wang W-H, Sung P-J. Discovery of Novel Diterpenoids from Sinularia arborea. Marine Drugs. 2014; 12(1):385-393. https://doi.org/10.3390/md12010385
Chicago/Turabian StyleChen, Kuan-Hua, Chang-Feng Dai, Tsong-Long Hwang, Chun-Yu Chen, Jan-Jung Li, Jih-Jung Chen, Yang-Chang Wu, Jyh-Horng Sheu, Wei-Hsien Wang, and Ping-Jyun Sung. 2014. "Discovery of Novel Diterpenoids from Sinularia arborea" Marine Drugs 12, no. 1: 385-393. https://doi.org/10.3390/md12010385





