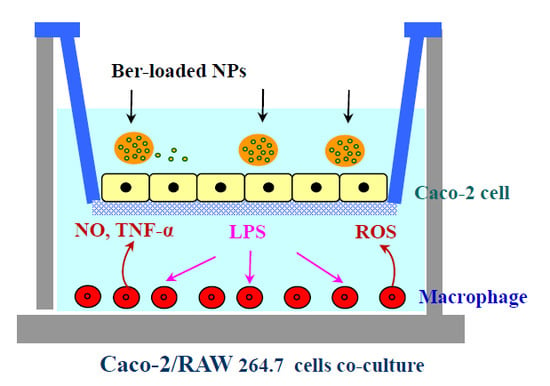
Delivery of Berberine Using Chitosan/Fucoidan-Taurine Conjugate Nanoparticles for Treatment of Defective Intestinal Epithelial Tight Junction Barrier
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of FD-Tau Conjugate


2.2. Characterization of Berberine-Loaded Nanoparticles
| FD-Tau (mg/mL) | Ber/FD-Tau Weight Ratio | Average Size (nm) | Zeta Potential (mV) | Drug Loading (%) | PDI |
|---|---|---|---|---|---|
| 0.5 | 2/1 | 111.7 ± 2.7 | –14.7 ± 1.4 | 10.3 ± 0.4 | 0.41 ± 0.01 |
| 1.0 | 2/2 | 120.9 ± 4.1 | –20.4 ± 0.7 | 13.1 ± 0.5 | 0.39 ± 0.02 |
| 1.5 | 2/3 | 147.4 ± 4.8 | –27.1 ± 0.9 | 9.7 ± 0.8 | 0.47 ± 0.01 |
| 2.0 | 2/4 | 156.3 ± 5.5 | –31.4 ± 1.8 | 12.8 ± 0.3 | 0.46 ± 0.02 |
| CS/Ber (mg/mg) | CS/Ber/FD-Tau Weight Ratio | Average Particle Size (nm) | PDI | Zeta Potential (mV) | Ber-Loading Content (%) |
|---|---|---|---|---|---|
| CS/FD-Tau NPs | |||||
| 0.5/0.0 | 1/0/4 | 225.6 ± 3.4 | 0.32 ± 0.02 | −38.2 ± 1.4 | − |
| 1.0/0.0 | 2/0/4 | 208.1 ± 5.5 | 0.35 ± 0.02 | −35.7 ± 2.1 | − |
| 1.5/0.0 | 3/0/4 | 204.5 ± 4.2 | 0.29 ± 0.02 | −11.7 ± 1.7 | − |
| 2.0/0.0 | 4/0/4 | 179.7 ± 3.9 | 0.33 ± 0.02 | +17.1 ± 1.6 | − |
| Ber-loaded CS/FD-Tau NPs | |||||
| 0.5/1.0 | 1/2/4 | 145.9 ± 2.7 | 0.27 ± 0.01 | −13.1 ± 0.8 | 32.3 ± 0.4 |
| 1.0/1.0 | 2/2/4 | 187.4 ± 6.2 | 0.21 ± 0.01 | +7.6 ± 0.5 | 50.1 ± 2.5 |
| 1.5/1.0 | 3/2/4 | 359.4 ± 4.7 | 0.39 ± 0.02 | +15.6 ± 0.6 | 62.7 ± 3.4 |
| 2.0/1.0 | 4/2/4 * | − | − | − | − |

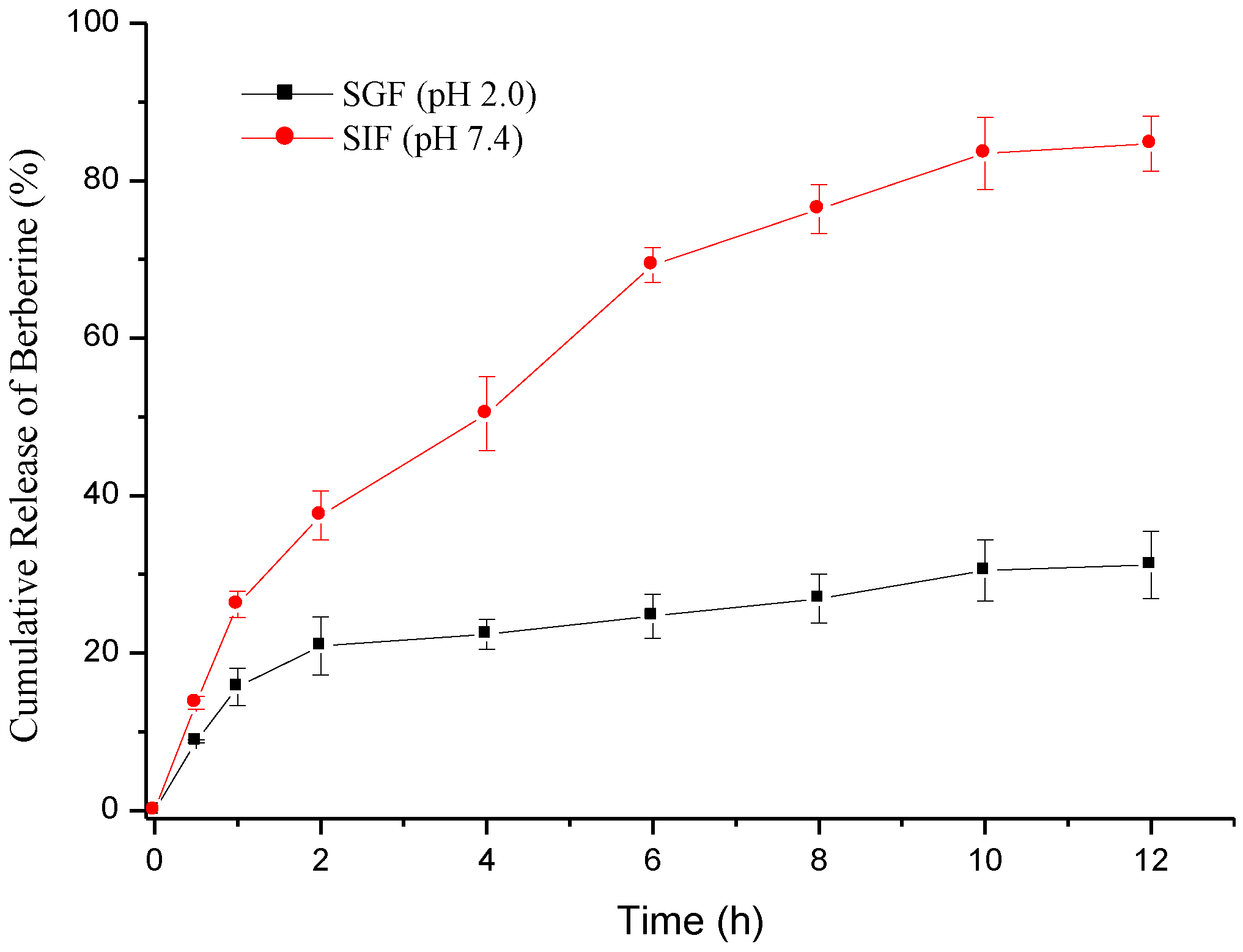
2.3. Berberine Release
2.4. Cytotoxicity of Berberine and Nanoparticles

2.5. Inhibition of NO and TNF-α Production

2.6. Intestinal TJ Permeability

2.7. Immunostaining of ZO-1 Protein

3. Experimental Section
3.1. Materials
3.2. Synthesis and Characterization of Fucoidan-Taurine (FD-Tau) Conjugates
3.3. Preparation of Berberine-Loaded CS/FD-Tau Nanoparticles
3.4. In Vitro Release Study
3.5. MTT Assay for Cell Viability
3.6. Caco-2/Macrophage Co-Culture System
3.7. Colorimetric Nitric Oxide Assay
3.8. Enzyme-Linked Immunosorbent Assay (ELISA) for TNF-α
3.9. Measurement of TEER and Paracellular Permeability
3.10. CLSM Visualization of Immunostained TJ Protein
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef]
- Amin, A.H.; Subbaiah, T.V.; Abbasi, K.M. Berberine sulfate: Antimicrobial activity, bioassay, and mode of action. Can. J. Microbiol. 1969, 15, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Wang, X.M.; Jiang, T.; Gong, J.F.; Niu, L.Y.; Li, N. Berberine prevents damage to the intestinal mucosal barrier during early phase of sepsis in rat through mechanisms independent of the NOD-like receptors signaling pathway. Eur. J. Pharmacol. 2014, 730, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, P.; Sun, C.; He, W.; Wang, F. Amelioration of IFN-gamma and TNF-alpha-induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway. PLoS One 2013, 8, e61944. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, N.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J. Infect. Dis. 2011, 203, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Amasheh, M.; Fromm, A.; Krug, S.M.; Amasheh, S.; Andres, S.; Zeitz, M.; Fromm, M.; Schulzke, J.D. TNFα-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFκB signaling. J. Cell Sci. 2010, 123, 4145–4155. [Google Scholar] [CrossRef]
- Li, N.; Gu, L.; Qu, L.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine attenuates pro-inflammatory cytokine-induced tight junction disruption in an in vitro model of intestinal epithelial cells. Eur. J. Pharmacol. Sci. 2010, 40, 1–8. [Google Scholar] [CrossRef]
- Tan, X.S.; Ma, J.Y.; Feng, R.; Ma, C.; Chen, W.J.; Sun, Y.P.; Fu, J.; Huang, M.; He, C.Y.; Shou, J.W.; et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS One 2013, 8, e77969. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Huang, W.Y.; Lai, C.H.; Hsu, Y.M.; Yao, Y.H.; Chen, T.Y.; Wu, J.Y.; Peng, S.F.; Lin, Y.H. Development of novel nanoparticles shelled with heparin for berberine delivery to treat Helicobacter pylori. Acta. Biomater. 2011, 7, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, J.H.; Chou, S.C.; Chang, S.J.; Chung, C.C.; Chen, Y.S.; Chang, C.H. Berberine-loaded targeted nanoparticles as specific Helicobacter pylori eradication therapy: In vitro and in vivo study. Nanomedicine 2014, 1, 1–15. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar. Drugs 2010, 8, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Sashiwa, H.; Aiba, S.I. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Harris, R.; Lecumberri, E.; Heras, A. Chitosan-genipin microspheres for the controlled release of drugs: Clarithromycin, tramadol and heparin. Mar. Drugs 2010, 8, 1750–1762. [Google Scholar] [CrossRef] [PubMed]
- Eftaiha, A.F.; Qinna, N.; Rashid, I.S.; Al Remawi, M.M.; Al Shami, M.R.; Arafat, T.A.; Badwan, A.A. Bioadhesive controlled metronidazole release matrix based on chitosan and xanthan gum. Mar. Drugs 2010, 8, 1716–1730. [Google Scholar] [CrossRef] [PubMed]
- Kavianinia, I.; Plieger, P.G.; Kandile, N.G.; Harding, D.R.K. In vitro evaluation of spray-dried chitosan microspheres crosslinked with pyromellitic dianhydride for oral colon-specific delivery of protein drugs. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Korkiatithaweechai, S.; Umsarika, P.; Praphairaksit, N.; Muangsin, N. Controlled release of diclofenac from matrix polymer of chitosan and oxidized konjac glucomannan. Mar. Drugs 2011, 9, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug. Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Nishitani, Y.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. In vitro model to estimate gut inflammation using co-cultured Caco-2 and RAW264.7 cells. Biochem. Biophys. Res. Commun. 2008, 374, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Iraha, A.; Chinen, H.; Hokama, A.; Yonashiro, T.; Kinjo, T.; Kishimoto, K.; Nakamoto, M.; Hirata, T.; Kinjo, N.; Higa, F.; et al. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J. Gastroenterol. 2013, 19, 5500–5507. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Liu, T.J. Mobilization of mesenchymal stem cells by stromal cell-derived factor-1 released from chitosan/tripolyphosphate/fucoidan nanoparticles. Acta Biomater. 2012, 8, 1048–1056. [Google Scholar] [PubMed]
- Yu, S.H.; Tang, D.W.; Hsieh, H.Y.; Wu, W.S.; Lin, B.X.; Chuang, E.Y.; Sung, H.W.; Mi, F.L. Nanoparticle-induced tight-junction opening for the transport of an anti-angiogenic sulfated polysaccharide across Caco-2 cell monolayers. Acta Biomater. 2013, 9, 7449–7459. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Li, R.Y. Preparation and characterization of antioxidant nanoparticles composed of chitosan and fucoidan for antibiotics delivery. Mar. Drugs 2014, 12, 4379–4398. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.C.; Garcia, T.; Mori, M.; Sandri, G.; Bonferoni, M.C.; Finotelli, P.V.; Cinelli, L.P.; Caramella, C.; Cabral, L.M. Preparation and characterization of polysaccharide-based nanoparticles with anticoagulant activity. Int. J. Nanomedicine 2012, 7, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Fa, Y.; Gu, B.; Zhu, W.; Zou, S. Taurine attenuates lipopolysaccharide-induced dysfunction in mouse mammary epithelial cells. Cytokine 2012, 59, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Kang, Y.J.; Park, M.K.; Lee, Y.S.; Seo, H.G.; Kim, T.S.; Kim, C.H.; Chang, K.C. Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-alpha, iNOS, and IL-12 production in LPS-stimulated macrophages. Life Sci. 2003, 73, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Hsu, K.C.; Lee, J.W.; Ham, M.; Huh, J.Y.; Shin, H.J.; Kim, W.S.; Kim, J.B. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E955–E964. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Gao, X.; Wang, Y.; Chen, H.; He, B.; Xu, H.; Li, Y.; Han, J.; Zhang, Z. Synthesis of glycyrrhetinic acid-modified chitosan 5-fluorouracil nanoparticles and its inhibition of liver cancer characteristics in vitro and in vivo. Mar. Drugs 2013, 11, 3517–3536. [Google Scholar] [CrossRef] [PubMed]
- Van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur. J. Pharm. Sci. 2001, 14, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Pyo, S.; Sohn, E.H. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-alpha- and IFN-gamma-stimulated C6 glioma cells. J. Nutr. Biochem. 2010, 21, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Nishitani, Y.; Hashimoto, T.; Kanazawa, K. Different suppressive effects of fucoidan and lentinan on IL-8 mRNA expression in in vitro gut inflammation. Biosci. Biotechnol. Biochem. 2009, 73, 2324–2325. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Satsu, H.; Nakano, T.; Shimizu, M. Regulation of the human taurine transporter by TNF-alpha and an anti-inflammatory function of taurine in human intestinal Caco-2 cells. BioFactors 2004, 21, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Mazzon, E.; de Sarro, A.; Caputi, A.P. Role of free radicals and poly(ADP-ribose) synthetase in intestinal tight junction permeability. Mol. Med. 2000, 6, 766–778. [Google Scholar] [PubMed]
- Rao, R.K.; Baker, R.D.; Baker, S.S.; Gupta, A.; Holycross, M. Oxidant-induced disruption of intestinal epithelial barrier function: Role of protein tyrosine phosphorylation. Am. J. Physiol. 1997, 273, G812–G823. [Google Scholar] [PubMed]
- Yu, S.H.; Mi, F.L.; Pang, J.C.; Jiang, S.C.; Kuo, T.H.; Wu, S.J.; Shyu, S.S. Preparation and characterization of radical and pH-responsive chitosan–gallic acid conjugate drug carriers. Carbohydr. Polym. 2011, 84, 794–802. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-J.; Don, T.-M.; Lin, C.-W.; Mi, F.-L. Delivery of Berberine Using Chitosan/Fucoidan-Taurine Conjugate Nanoparticles for Treatment of Defective Intestinal Epithelial Tight Junction Barrier. Mar. Drugs 2014, 12, 5677-5697. https://doi.org/10.3390/md12115677
Wu S-J, Don T-M, Lin C-W, Mi F-L. Delivery of Berberine Using Chitosan/Fucoidan-Taurine Conjugate Nanoparticles for Treatment of Defective Intestinal Epithelial Tight Junction Barrier. Marine Drugs. 2014; 12(11):5677-5697. https://doi.org/10.3390/md12115677
Chicago/Turabian StyleWu, Shao-Jung, Trong-Ming Don, Cheng-Wei Lin, and Fwu-Long Mi. 2014. "Delivery of Berberine Using Chitosan/Fucoidan-Taurine Conjugate Nanoparticles for Treatment of Defective Intestinal Epithelial Tight Junction Barrier" Marine Drugs 12, no. 11: 5677-5697. https://doi.org/10.3390/md12115677