Co-Cultivation—A Powerful Emerging Tool for Enhancing the Chemical Diversity of Microorganisms
Abstract
:1. Introduction
2. Results
2.1. Co-Cultivation Studies of Marine-Derived Microorganisms with Influence on Natural Product Accumulation
| Co-Cultivated Microorganisms | Secondary Metabolites Reported | Reported Activity | Reference |
|---|---|---|---|
| Aspergillus sp. Aspergillus sp. | Aspergicin (n, 1) Neoaspergillic acid (k) Ergosterol (k) | Antibiotic Antibiotic - | [48] |
| Unidentified fungus Unidentified fungus | 8-Hydroxy-3-methyl-9-oxo-9H-xanthene-1-carboxylic acid methylether (n, 2) | Antifungal | [49] |
| Unidentified fungus Unidentified fungus | Marinamide (n, 3) Marinamide methylether (n, 4) | Cytotoxic Cytotoxic | [60] |
| Pestalotia sp. Unidentified bacterium | Pestalone (n, 5) | - | [52] |
| Libertella sp. Thalassopia sp. | Libertellenones A–D (n, 6–9) | Cytotoxic | [53] |
| Emericella ap. Salinospora arenicola | Emericellamide A (n, 10) Emericellamide B (n, 11) | Antibiotic Cytotoxic | [54] |
| Aspergillus fumigatus Sphingomonas sp. | Glionitrin A (n, 12) | Cytotoxic Antibiotic | [55] |
| B. thuringensis B. Megaterium S. sciuri | Indole (k) Phe-Pro diketopiperazine (k) | Antibiotic (on extract level) | [59] |
| Streptomyces tenjimariensis 12 unidentified bacteria | Istamycin (k) | Antibiotic | [34] |
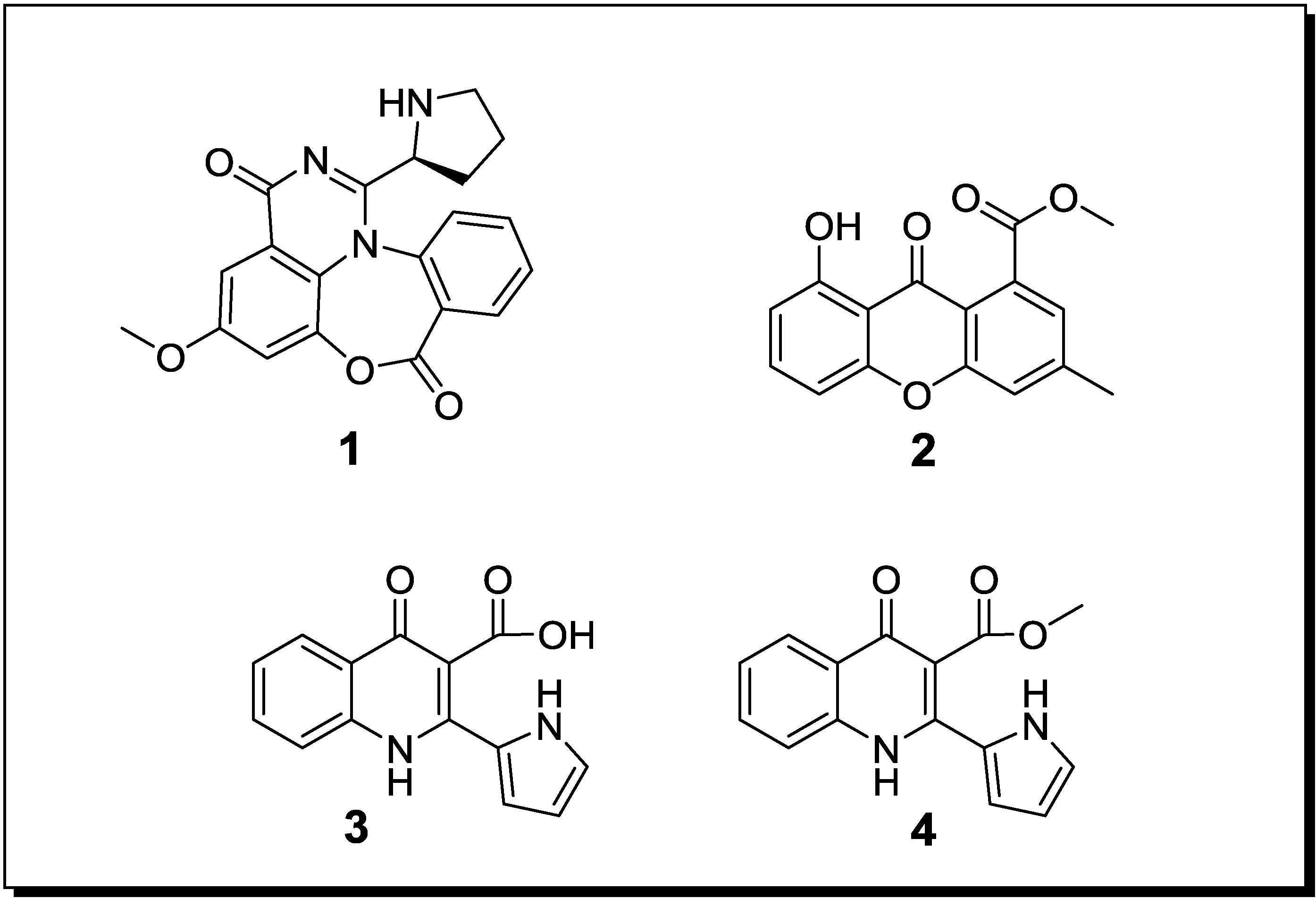
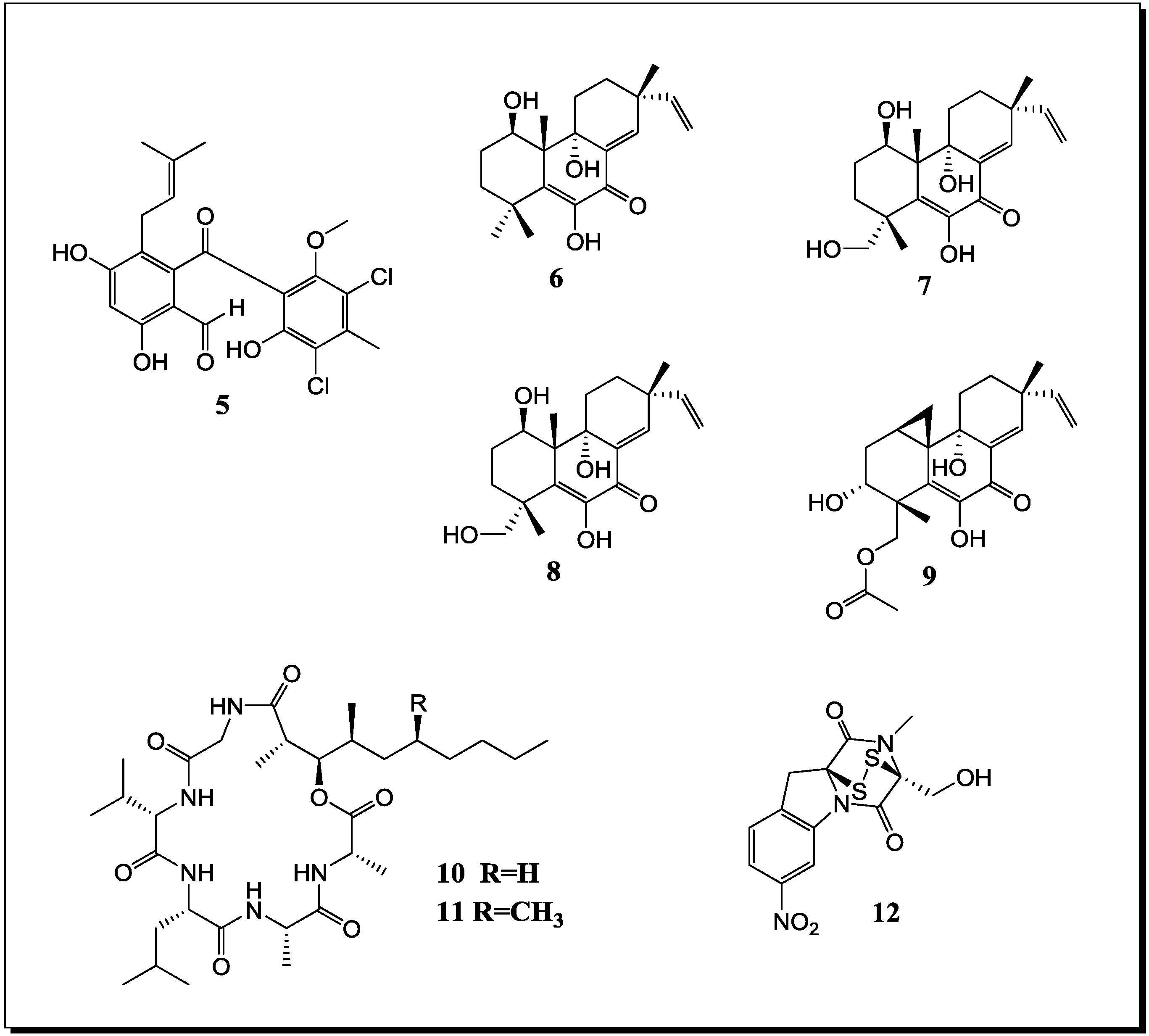
2.2. Co-Cultivation Studies of Terrestrial Microorganisms with Influence on Natural Product Accumulation
| Co-Cultivated Microorganisms | Secondary Metabolites Reported | Reported Activity | Reference |
|---|---|---|---|
| Penicillium pinophilum Trichoderma harzianum | Secopenicillide C (n, 13) | n.t. | [61] |
| Penicillide (k) | n.t. | ||
| MC-141 (k) | n.t. | ||
| Pestalasin A (k) | n.t. | ||
| Stromemycin (k) | n.t. | ||
| Fusarium tricinctum Fusarium begoniae | Subenniatin A (n, 14) | - | [62] |
| Subenniatin B (n, 15) | - | ||
| Enniatin A (k) | - | ||
| Enniatin A1 (k) | - | ||
| Enniatin B (k) | - | ||
| Enniatin B1 (k) | - | ||
| Acremonium sp. Mycogone rosea | Acremostatin A (n, 16) | n.t. | [63] |
| Acremostatin B (n, 17) | n.t. | ||
| Acremostatin C (n, 18) | n.t. | ||
| Gloeophyllum abietinum Heterobasidion annosum Armillaria ostoyae | Oospoglycol (k) | n.t. | [64] |
| Oopsonol (k) | n.t. | ||
| Fomannoxin (k) | n.t. | ||
| Fomannoxinalcohol (k) | n.t. | ||
| Fomannosin (k) | n.t. | ||
| Melledonal (k) | n.t. | ||
| Melledonal C (k) | n.t. | ||
| Melleolide D (k) | n.t. | ||
| Oyadendron sulphureoochraceum Ascochyta pisi Emercillopsis minima Cylindrocarpon destructans Fusarium oxysporum | Lateritin (k) | Cytotoxic Antifungal Antibiotic | [65] |
| Paraconiothyrium sp. Alternaria sp. Phomopsis sp. | Paclitaxel (k) | n.t. | [66] |
| Streptomyces bullii Aspergillus fumigatus | Brevianamide F (k) | -/-/C | [67] |
| Spirotryprostatin A (k) | T/L/C | ||
| 6-Methoxy spirotrypostatin B (k) | -/L/C | ||
| Fumitremorgin C (k) | T/L/C | ||
| 12,13-Dihydroxy Fumitremorgin C (k) | T/L/C | ||
| Fumitremorgin B (k) | T/L/C | ||
| Verruculogen (k) | T/L/C | ||
| 11-O-Methylpseurotin A (k) | -/-/C | ||
| 11-O-Methylpseurotin A2 (n, 19) | -/L/C | ||
| Ergosterol (k) | -/-/- | ||
| Emestrin A (k) | n.t. | ||
| Emestrin B (k) | n.t. | ||
| Aspergillus fumigatus Streptomyces rapamycinicus | Fumicycline A (n, 20) | Antibiotic | [45] |
| Fumicyline B (n, 21) | Antibiotic | ||
| Aspergillus fumigatus Streptomyces peucetius | Fumiformamide (n, 22) NN′-((1Z,3Z)-1,4-bis (4-Methoxyphenyl)buta-1,3-diene-2,3diyl)Di-formamide (n, 23) | Cytotoxic | [68] |
| Fusarium tricinctum Bacillus subtilis | Macrocarpon C (n, 24) | - | [30] |
| 2-(Carboxymethylamino)benzoic acid (n, 25) | - | ||
| (−)-Citreoisocoumarinol (n, 26) | - | ||
| Lateropyrone (k) | Antibiotic | ||
| Enniatin A1 (k) | Antibiotic | ||
| Enniatin B (k) | - | ||
| Enniatin B1 (k) | Antibiotic | ||
| (+)-Citreoisocoumarinol (k) | - | ||
| Tsukamurella pulmonis Streptomyces endus | Alchivemycin A (n, 27) | Antibiotic | [69] |
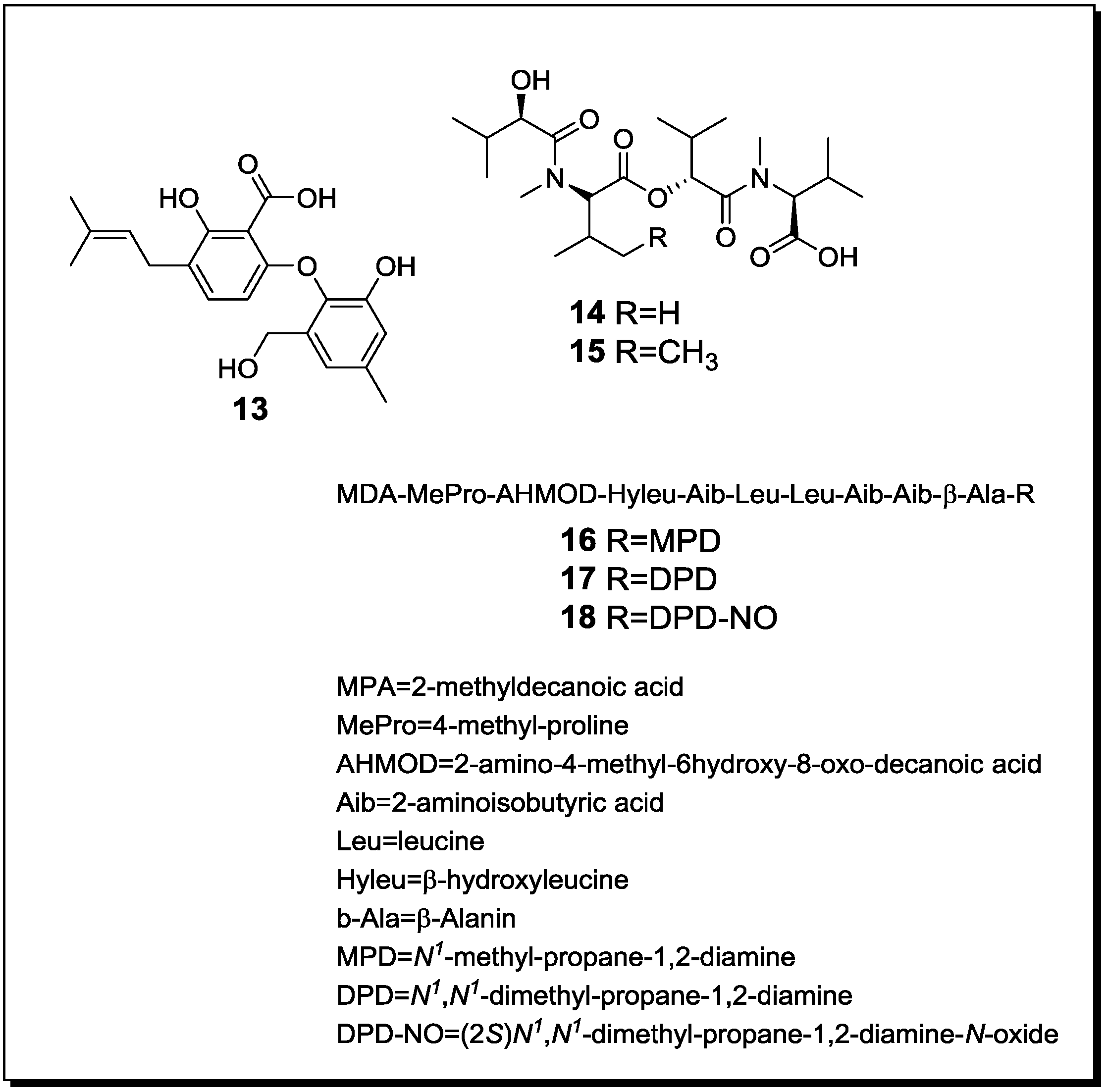
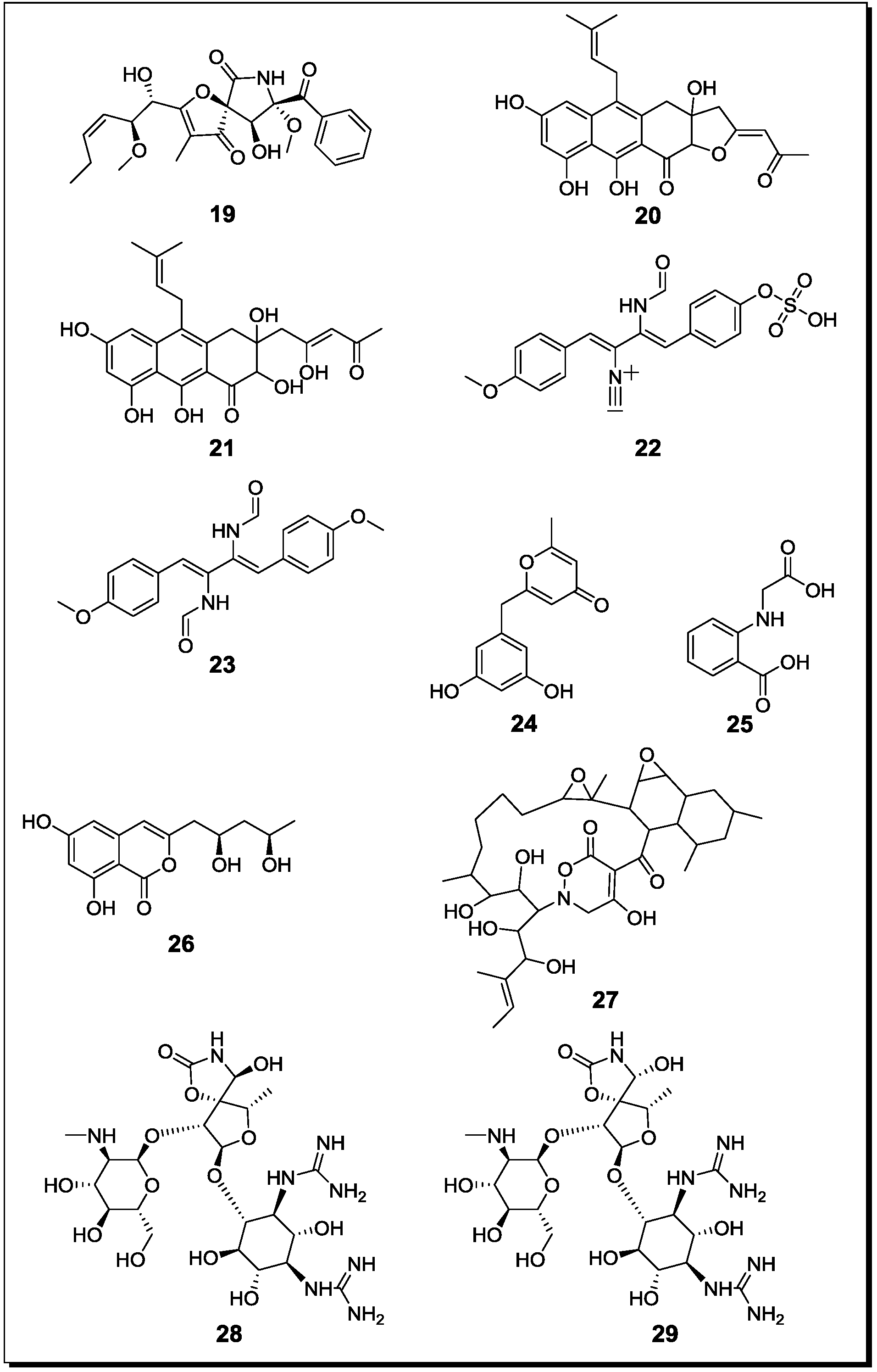
2.3. Molecular Biology and Accumulation of Cryptic Natural Products in Mixed Fermentations of the Model Organism Aspergillus Nidulans and Streptomyces Rapamycinicus
3. Discussion and Outlook
Conflicts of Interest
References
- Li, J.W.-H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Enomoto, T.; Yasui, Y.; Takemoto, Y. Synthetic study toward ecteinascidin 743: Concise construction of the diazabicyclo [3.3.1] nonane skeleton and assembly of the pentacyclic core. J. Org. Chem. 2010, 75, 4876–4879. [Google Scholar] [CrossRef]
- Younes, A.; Yasothan, U.; Kirkpatrick, P. Brentuximab vedotin. Nat. Rev. Drug Discov. 2012, 11, 19–20. [Google Scholar] [CrossRef]
- Reichert, J.M. Marketed Therapeutic Antibodies Compendium; MAbs-Landes Bioscience: Austin, TX, USA, 2012; pp. 413–415. [Google Scholar]
- European Medicines Agency: Adcetris. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002455/human_med_001588.jsp&mid=WC0b01ac058001d124 (accessed on 3 January 2014).
- Huyck, T.K.; Gradishar, W.; Manuguid, F.; Kirkpatrick, P. Eribulin mesylate. Nat. Rev. Drug Discovery 2011, 10, 173–174. [Google Scholar] [CrossRef]
- Mayer, A.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Glaser, K.B.; Mayer, A. A renaissance in marine pharmacology: From preclinical curiosity to clinical reality. Biochem. Pharmacol. 2009, 78, 440–448. [Google Scholar] [CrossRef]
- Schmidtko, A.; Lötsch, J.; Freynhagen, R.; Geisslinger, G. Ziconotide for treatment of severe chronic pain. Lancet 2010, 375, 1569–1577. [Google Scholar] [CrossRef]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef]
- Yasuhara-Bell, J.; Lu, Y. Marine compounds and their antiviral activities. Antivir. Res. 2010, 86, 231–240. [Google Scholar] [CrossRef]
- Serova, M.; de Gramont, A.; Bieche, I.; Riveiro, M.E.; Galmarini, C.M.; Aracil, M.; Jimeno, J.; Faivre, S.; Raymond, E. Predictive factors of sensitivity to elisidepsin, a novel kahalalide F-derived marine compound. Mar. Drugs 2013, 11, 944–959. [Google Scholar] [CrossRef]
- Barboza, N.M.; Medina, D.J.; Budak-Alpdogan, T.; Aracil, M.; Jimeno, J.M.; Bertino, J.R.; Banerjee, D. Plitidepsin (Aplidin) is a potent inhibitor of diffuse large cell and Burkitt lymphoma and is synergistic with rituximab. Cancer Biol. Ther. 2012, 13, 114–122. [Google Scholar] [CrossRef]
- Molina-Guijarro, J.M.; Macías, Á.; García, C.; Muñoz, E.; García-Fernández, L.F.; David, M.; Núñez, L.; Martínez-Leal, J.F.; Moneo, V.; Cuevas, C. Irvalec inserts into the plasma membrane causing rapid loss of integrity and necrotic cell death in tumor cells. PLoS One 2011, 6, e19042. [Google Scholar] [CrossRef] [Green Version]
- Marine pharmaceuticals: The clinical pipeline. Available online: http://marinepharmacology.midwestern.edu/clinPipeline.htm (accessed on 3 January 2014).
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem. Int. Ed. 2003, 42, 355–357. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.R.; Palladino, M.A.; Lam, K.S.; Lloyd, G.K.; Potts, B.C. Discovery and development of the anticancer agent salinosporamide A (NPI-0052). Bioorg. Med. Chem. 2009, 17, 2175–2180. [Google Scholar] [CrossRef]
- Chauhan, D.; Catley, L.; Li, G.; Podar, K.; Hideshima, T.; Velankar, M.; Mitsiades, C.; Mitsiades, N.; Yasui, H.; Letai, A. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell 2005, 8, 407–419. [Google Scholar] [CrossRef]
- Groll, M.; Huber, R.; Potts, B.C.M. Crystal structures of salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of β-lactone ring opening and a mechanism for irreversible binding. J. Am. Chem. Soc. 2006, 128, 5136–5141. [Google Scholar] [CrossRef]
- Knight, V.; Sanglier, J.J.; DiTullio, D.; Braccili, S.; Bonner, P.; Waters, J.; Hughes, D.; Zhang, L. Diversifying microbial natural products for drug discovery. Appl. Microbiol. Biotechnol. 2003, 62, 446–458. [Google Scholar] [CrossRef]
- Brakhage, A.A.; Schuemann, J.; Bergmann, S.; Scherlach, K.; Schroeckh, V.; Hertweck, C. Activation of Fungal Silent Gene Clusters: A New Avenue to Drug Discovery. In Natural Compounds as Drugs; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–12. [Google Scholar]
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites–strategies to activate silent gene clusters. Fungal Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Cichewicz, R.H. Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Nat. Prod. Rep. 2010, 27, 11–22. [Google Scholar] [CrossRef]
- Shwab, E.K.; Bok, J.W.; Tribus, M.; Galehr, J.; Graessle, S.; Keller, N.P. Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryotic Cell 2007, 6, 1656–1664. [Google Scholar] [CrossRef]
- Henrikson, J.C.; Hoover, A.R.; Joyner, P.M.; Cichewicz, R.H. A chemical epigenetics approach for engineering the in situ biosynthesis of a cryptic natural product from Aspergillus niger. Org. Biomol. Chem. 2009, 7, 435–438. [Google Scholar] [CrossRef]
- Fisch, K.M.; Gillaspy, A.F.; Gipson, M.; Henrikson, J.C.; Hoover, A.R.; Jackson, L.; Najar, F.Z.; Wägele, H.; Cichewicz, R.H. Chemical induction of silent biosynthetic pathway transcription in Aspergillus niger. J. Ind. Microbiol. Biotechnol. 2009, 36, 1199–1213. [Google Scholar] [CrossRef]
- Asai, T.; Chung, Y.-M.; Sakurai, H.; Ozeki, T.; Chang, F.-R.; Yamashita, K.; Oshima, Y. Tenuipyrone, a novel skeletal polyketide from the entomopathogenic fungus, Isaria tenuipes, cultivated in the presence of epigenetic modifiers. Org. Lett. 2011, 14, 513–515. [Google Scholar]
- Vervoort, H.C.; Drašković, M.; Crews, P. Histone deacetylase inhibitors as a tool to up-regulate new fungal biosynthetic products: Isolation of EGM-556, a cyclodepsipeptide, from Microascus sp. Org. Lett. 2010, 13, 410–413. [Google Scholar]
- Ola, A.R.B.; Thomy, D.; Lai, D.; Brötz-Oesterhelt, H.; Proksch, P. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J. Nat. Prod. 2013, 76, 2094–2099. [Google Scholar] [CrossRef]
- Wiener, P. Experimental studies on the ecological role of antibiotic production in bacteria. Evol. Ecol. 1996, 10, 405–421. [Google Scholar] [CrossRef]
- Anke, T. The antifungal strobilurins and their possible ecological role. Can. J. Bot. 1995, 73, 940–945. [Google Scholar] [CrossRef]
- Williams, S.T.; Vickers, J.C. The ecology of antibiotic production. Microb. Ecol. 1986, 12, 43–52. [Google Scholar] [CrossRef]
- Slattery, M.; Rajbhandari, I.; Wesson, K. Competition-mediated antibiotic induction in the marine bacterium Streptomyces tenjimariensis. Microb. Ecol. 2001, 41, 90–96. [Google Scholar]
- De Lorenzo, V.; Aguilar, A. Antibiotics from Gram-negative bacteria: Do they play a role in microbial ecology? Trends Biochem. Sci. 1984, 9, 266–269. [Google Scholar] [CrossRef]
- Linares, J.F.; Gustafsson, I.; Baquero, F.; Martinez, J.L. Antibiotics as intermicrobial signaling agents instead of weapons. Sci. Signal. 2006, 103, 19484. [Google Scholar]
- Fajardo, A.; Martínez, J.L. Antibiotics as signals that trigger specific bacterial responses. Curr. Opin. Microbiol. 2008, 11, 161–167. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Zhu, Y.; Gong, Q.; Yu, W.; Lu, X. Antibiotics at subinhibitory concentrations improve the quorum sensing behavior of Chromobacterium violaceum. FEMS Microbiol. Lett. 2013, 341, 37–44. [Google Scholar] [CrossRef]
- Shang, Z.; Li, X.-M.; Li, C.-S.; Wang, B.-G. Diverse secondary metabolites produced by marine-derived fungus Nigrospora sp. MA75 on various culture media. Chem. Biodivers. 2012, 9, 1338–1348. [Google Scholar] [CrossRef]
- Ebrahim, W.; Aly, A.H.; Mándi, A.; Totzke, F.; Kubbutat, M.H.G.; Wray, V.; Lin, W.H.; Dai, H.; Proksch, P.; Kurtán, T.; et al. Decalactone derivatives from Corynespora cassiicola, an endophytic fungus of the mangrove plant Laguncularia racemosa. Eur. J. Org. Chem. 2012, 2012, 3476–3484. [Google Scholar] [CrossRef]
- Xu, J.; Aly, A.H.; Wray, V.; Proksch, P. Polyketide derivatives of endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Tetrahedron Lett. 2011, 52, 21–25. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Volkmann-Kohlmeyer, B.; Kohlmeyer, J. Independent terrestrial origins of the Halosphaeriales (marine Ascomycota). Am. J. Bot. 1998, 85, 1569–1580. [Google Scholar] [CrossRef]
- Raghukumar, C. Marine fungal biotechnology: An ecological perspective. Fungal Divers. 2008, 31, 19–35. [Google Scholar]
- Kohlmeyer, J.; Kohlmeyer, E. Marine Mycology. The Higher Fungi; Academic Press, Inc.: Waltham, MA, USA, 1979. [Google Scholar]
- König, C.C.; Scherlach, K.; Schroeckh, V.; Horn, F.; Nietzsche, S.; Brakhage, A.A.; Hertweck, C. Bacterium induces cryptic meroterpenoid pathway in the pathogenic fungus Aspergillus fumigatus. ChemBioChem 2013, 14, 938–942. [Google Scholar] [CrossRef]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.-W.; Shelest, E.; Schmidt-Heck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate bacterial–fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef]
- Nützmann, H.-W.; Reyes-Dominguez, Y.; Scherlach, K.; Schroeckh, V.; Horn, F.; Gacek, A.; Schümann, J.; Hertweck, C.; Strauss, J.; Brakhage, A.A. Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc. Natl. Acad. Sci. USA 2011, 108, 14282–14287. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, G.; Chen, X.; Huang, M.; Wan, X. Aspergicin, a new antibacterial alkaloid produced by mixed fermentation of two marine-derived mangrove epiphytic fungi. Chem. Nat. Compd. 2011, 47, 767–769. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Shao, C.; Ding, W.; She, Z.; Lin, Y. A new xanthone derivative from the co-culture broth of two marine fungi (strain No. E33 and K38). Chem. Nat. Compd. 2011, 47, 382–384. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, G.; Wu, J.; Pan, J. Structure revision and cytotoxic activity of marinamide and its methyl ester, novel alkaloids produced by co-cultures of two marine-derived mangrove endophytic fungi. Nat. Prod. Res. 2013, 27, 1960–1964. [Google Scholar] [CrossRef]
- Miao, L.I.; Kwong, T.F.N.; Qian, P.-Y. Effect of culture conditions on mycelial growth, antibacterial activity, and metabolite profiles of the marine-derived fungus Arthrinium c.f. saccharicola. Appl. Microbiol. Biotechnol. 2006, 72, 1063–1073. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Oh, D.-C.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Libertellenones A–D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorg. Med. Chem. 2005, 13, 5267–5273. [Google Scholar] [CrossRef]
- Oh, D.-C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J. Nat. Prod. 2007, 70, 515–520. [Google Scholar] [CrossRef]
- Park, H.B.; Kwon, H.C.; Lee, C.-H.; Yang, H.O. Glionitrin A, an antibiotic-antitumor metabolite derived from competitive interaction between abandoned mine microbes. J. Nat. Prod. 2009, 72, 248–252. [Google Scholar] [CrossRef]
- Dusane, D.H.; Matkar, P.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Cross-species induction of antimicrobial compounds, biosurfactants and quorum-sensing inhibitors in tropical marine epibiotic bacteria by pathogens and biofouling microorganisms. Curr. Microbiol. 2011, 62, 974–980. [Google Scholar] [CrossRef]
- Burgess, J.G.; Jordan, E.M.; Bregu, M.; Mearns-Spragg, A.; Boyd, K.G. Microbial antagonism: A neglected avenue of natural products research. Prog. Ind. Microbiol. 1999, 35, 27–32. [Google Scholar] [CrossRef]
- Mearns-Spragg, A.; Bregu, M.; Boyd, K.G.; Burgess, J.G. Cross-species induction and enhancement of antimicrobial activity produced by epibiotic bacteria from marine algae and invertebrates, after exposure to terrestrial bacteria. Lett. Appl. Microbiol. 1998, 27, 142–146. [Google Scholar]
- Trischman, J.A.; Oeffner, R.E.; de Luna, M.G.; Kazaoka, M. Competitive induction and enhancement of indole and a diketopiperazine in marine bacteria. Mar. Biotechnol. 2004, 6, 215–220. [Google Scholar]
- Zhu, F.; Lin, Y. Marinamide, a novel alkaloid and its methyl ester produced by the application of mixed fermentation technique to two mangrove endophytic fungi from the South China Sea. Chin. Sci. Bull. 2006, 51, 1426–1430. [Google Scholar] [CrossRef]
- Nonaka, K.; Abe, T.; Iwatsuki, M.; Mori, M.; Yamamoto, T.; Shiomi, K.; Ômura, S.; Masuma, R. Enhancement of metabolites productivity of Penicillium pinophilum FKI-5653, by co-culture with Trichoderma harzianum FKI-5655. J. Antibiot. 2011, 64, 769–774. [Google Scholar] [CrossRef]
- Wang, J.-P.; Lin, W.; Wray, V.; Lai, D.; Proksch, P. Induced production of depsipeptides by co-culturing Fusarium tricinctum and Fusarium begoniae. Tetrahedron Lett. 2013, 54, 2492–2496. [Google Scholar] [CrossRef]
- Degenkolb, T.; Heinze, S.; Schlegel, B.; Strobel, G.; Gräfe, U. Formation of new lipoaminopeptides, acremostatins A, B, and C, by co-cultivation of Acremonium sp. Tbp-5 and Mycogone rosea DSM 12973. Biosci. Biotechnol. Biochem. 2002, 66, 883–886. [Google Scholar] [CrossRef]
- Sonnenbichler, J.; Dietrich, J.; Peipp, H. Secondary fungal metabolites and their biological activities, V. Investigations concerning the induction of the biosynthesis of toxic secondary metabolites in basidiomycetes. Bio. Chem. Hoppe-Seyler 1994, 375, 71–80. [Google Scholar] [CrossRef]
- Pettit, R.K.; Pettit, G.R.; Xu, J.P.; Weber, C.A.; Richert, L.A. Isolation of human cancer cell growth inhibitory, antimicrobial lateritin from a mixed fungal culture. Planta Med. 2010, 76, 500–501. [Google Scholar] [CrossRef]
- Soliman, S.S.M.; Raizada, M.N. Interactions between co-habitating fungi elicit synthesis of taxol from an endophytic fungus in host Taxus plants. Front. Microbiol. 2013, 4, 1–14. [Google Scholar]
- Rateb, M.E.; Hallyburton, I.; Houssen, W.; Bull, A.; Goodfellow, M.; Santhanam, R.; Jaspars, M.; Ebel, R. Induction of diverse secondary metabolites in Aspergillus fumigatus by microbial co-culture. RSC Adv. 2013, 3, 14444–14450. [Google Scholar] [CrossRef] [Green Version]
- Zuck, K.M.; Shipley, S.; Newman, D.J. Induced production of N-formyl alkaloids from Aspergillus fumigatus by co-culture with Streptomyces peucetius. J. Nat. Prod. 2011, 74, 1653–1657. [Google Scholar] [CrossRef]
- Onaka, H.; Mori, Y.; Igarashi, Y.; Furumai, T. Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species. Appl. Environ. Microbiol. 2011, 77, 400–406. [Google Scholar] [CrossRef]
- Shin, C.S.; Kim, H.J.; Kim, M.J.; Ju, J.Y. Morphological change and enhanced pigment production of Monascus when cocultured with Saccharomyces cerevisiae or Aspergillus oryzae. Biotechnol. Bioeng. 1998, 59, 576–581. [Google Scholar] [CrossRef]
- Gibson, J.; Sood, A.; Hogan, D.A. Pseudomonas aeruginosa-Candida albicans interactions: Localization and fungal toxicity of a phenazine derivative. Appl. Environ. Microbiol. 2009, 75, 504–513. [Google Scholar] [CrossRef]
- Turpin, P.E.; Dhir, V.K.; Maycroft, K.A.; Rowlands, C.; Wellington, E.M.H. The effect of Streptomyces species on the survival of Salmonella in soil. FEMS Microbiol. Lett. 1992, 101, 271–280. [Google Scholar]
- Zhou, Y.; Debbab, A.; Mándi, A.; Wray, V.; Schulz, B.; Müller, W.E.G.; Kassack, M.; Lin, W.; Kurtán, T.; Proksch, P. Alkaloids from the sponge-associated fungus Aspergillus sp. Eur. J. Org. Chem. 2013, 2013, 894–906. [Google Scholar]
- Brauers, G.; Edrada, R.A.; Ebel, R.; Proksch, P.; Wray, V.; Berg, A.; Gräfe, U.; Schächtele, C.; Totzke, F.; Finkenzeller, G. Anthraquinones and betaenone derivatives from the sponge-associated fungus Microsphaeropsis species: Novel inhibitors of protein kinases. J. Nat. Prod. 2000, 63, 739–745. [Google Scholar] [CrossRef]
- Yang, L.H.; Miao, L.; Lee, O.O.; Li, X.; Xiong, H.; Pang, K.-L.; Vrijmoed, L.; Qian, P.-Y. Effect of culture conditions on antifouling compound production of a sponge-associated fungus. Appl. Microbiol. Biotechnol. 2007, 74, 1221–1231. [Google Scholar] [CrossRef]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef]
- Mitova, M.; Tommonaro, G.; Hentschel, U.; Müller, W.E.G.; de Rosa, S. Exocellular cyclic dipeptides from a Ruegeria strain associated with cell cultures of Suberites domuncula. Mar. Biotechnol. 2004, 6, 95–103. [Google Scholar] [CrossRef]
- König, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Müller, D. Natural products from marine organisms and their associated microbes. ChemBioChem 2006, 7, 229–238. [Google Scholar] [CrossRef]
- Zuccaro, A.; Schoch, C.L.; Spatafora, J.W.; Kohlmeyer, J.; Draeger, S.; Mitchell, J.I. Detection and identification of fungi intimately associated with the brown seaweed Fucus serratus. Appl. Environ. Microbiol. 2008, 74, 931–941. [Google Scholar] [CrossRef]
- Berland, B.R.; Bonin, D.J.; Maestrini, S.Y. Study of bacteria associated with marine algae in culture. Mar. Biol. 1970, 5, 68–76. [Google Scholar] [CrossRef]
- Wiese, J.; Thiel, V.; Nagel, K.; Staufenberger, T.; Imhoff, J.F. Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the Baltic Sea. Mar. Biotechnol. 2009, 11, 287–300. [Google Scholar] [CrossRef] [Green Version]
- Amrani, M.E.; Debbab, A.; Aly, A.H.; Wray, V.; Dobretsov, S.; Müller, W.E.G.; Lin, W.; Lai, D.; Proksch, P. Farinomalein derivatives from an unidentified endophytic fungus isolated from the mangrove plant Avicennia marina. Tetrahedron Lett. 2012, 53, 6721–6724. [Google Scholar] [CrossRef]
- Kjer, J.; Wray, V.; Edrada-Ebel, R.; Ebel, R.; Pretsch, A.; Lin, W.; Proksch, P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J. Nat. Prod. 2009, 72, 2053–2057. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Winans, S.C.; Holguin, G. Molecular characterization of diazotrophic and denitrifying bacteria associated with mangrove roots. Appl. Environ. Microbiol. 2007, 73, 7308–7321. [Google Scholar] [CrossRef]
- Díaz, M.P.; Boyd, K.G.; Grigson, S.J.W.; Burgess, J.G. Biodegradation of crude oil across a wide range of salinities by an extremely halotolerant bacterial consortium MPD-M, immobilized onto polypropylene fibers. Biotechnol. Bioeng. 2002, 79, 145–153. [Google Scholar] [CrossRef]
- Kurosawa, K.; Ghiviriga, I.; Sambandan, T.G.; Lessard, P.A.; Barbara, J.E.; Rha, C.; Sinskey, A.J. Rhodostreptomycins, antibiotics biosynthesized following horizontal gene transfer from Streptomyces padanus to Rhodococcus fascians. J. Am. Chem. Soc. 2008, 130, 1126–1127. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-Cultivation—A Powerful Emerging Tool for Enhancing the Chemical Diversity of Microorganisms. Mar. Drugs 2014, 12, 1043-1065. https://doi.org/10.3390/md12021043
Marmann A, Aly AH, Lin W, Wang B, Proksch P. Co-Cultivation—A Powerful Emerging Tool for Enhancing the Chemical Diversity of Microorganisms. Marine Drugs. 2014; 12(2):1043-1065. https://doi.org/10.3390/md12021043
Chicago/Turabian StyleMarmann, Andreas, Amal H. Aly, Wenhan Lin, Bingui Wang, and Peter Proksch. 2014. "Co-Cultivation—A Powerful Emerging Tool for Enhancing the Chemical Diversity of Microorganisms" Marine Drugs 12, no. 2: 1043-1065. https://doi.org/10.3390/md12021043





