The Challenge of Ecophysiological Biodiversity for Biotechnological Applications of Marine Microalgae
Abstract
:1. Introduction
2. Biotechnological Applications of Microalgae and Cultivation
2.1. Biotechnological Applications of Microalgae
2.2. Algal Cultivation Techniques
3. Exploring the Richness of Microalgal Biodiversity
3.1. Taxonomical Diversity
3.2. Functional Diversity: Morphology and Size
| Application | Function | Requirements | Algae |
|---|---|---|---|
| Biomass production | Fluxes of matter and energy | Optimization of culture conditions for growth and photosynthesis maximization | Large-sized coastal species, species with a high constitutive growth and photosynthesis |
| Primary metabolites | Production of interesting molecules such as carotenoids, phycobiliproteins, proteins, lipids, polysaccharides and antioxidants | Optimization of culture conditions for maximizing interesting molecules production and high growth rates (photosynthetic biotechnology through light manipulation and metabolic engineering) | Physiologically plastic species, such as small diatoms and coastal species |
| Secondary metabolites | Production of toxin or drugs | Optimal or stressful conditions to produce this kind of molecules | Selected or genetically modified species |
| Functional trait or adaptive feature | Ecological or physiological relevance | Group of species | Interests and/or problems in biotechnology |
|---|---|---|---|
| Multicellular life forms (chains and colony; [117]) | Influence of sinking rate, reduced predation | Diatoms, haptophytes | Little impact in shallow and oxygenated/mixed tanks |
| Flagellates [131] | Migration and motility | Dinophytes, haptophytes and cryptophytes | Little impact in shallow and oxygenated/mixed tanks |
| Small cell size [127] | Low nutrient requirements, high growth capacity, low sinking rate | Picoeukaryotes | High growth and production capacity and acclimation |
| Benthic species [26,132] | Growth on solid support (sediments, leaves), highly resistant species | Some diatoms, cyanophytes | Difficult to cultivate |
| Toxic species [133] | Defence mechanisms, highly competitive | Cyanophytes, diatoms, dinophytes | Discovery and selection of new molecules |
| Sexual reproduction [134] | Genetic recombination | Some diatoms | New strains selection with better fitness |
| Diazotrophy [135] | Atmospheric N2 fixation | Cyanophytes | Low growth capacity |
| Mixotrophy [136,137,138] | Growth under nutrients depletion and darkness | Some dinophytes, diatoms, chrysophytes and cryptophytes | Low growth capacity, interest for bioremediation |
| Presence of large vacuoles [102] | Internal storage of nutrient | Diatoms | Long-term maintenance, decrease of dilution frequency |
| Low light adapted [139] | Growth under low light, photoinhibited under high light | Deep chlorophyll maximum (DCM) species | High growth rate under low light, high capacity of photoprotection |
| Variable light adapted [24] | Growth under low and high light, physiological plasticity | Coastal species (some diatoms and haptophytes) | High capacity of xanthophyll and antioxidant production, high growth rate |
| Low iron requirement species [140] | Growth in pelagic/oceanic ecosystems, photobiological and physiological adaptation | Some pennate diatoms | Little effect, Fe is provided in high quantity |
| Oceanic Temperature zones [141,142] | Low temperature growth | Polar species (diatoms, haptophytes) | Cost for low temperature maintenance of the cultures Temperature defence mechanisms, peculiar molecules for allowing photosynthesis and production at low temperature |
| Calcareous microalgae [143] | Species producing calcified scales around the cell | Coccolithophorids | Calcite production |
| DMSP, DMS producer species [15] | Antioxidant production (* DMS and ** DMSP) under environmental stresses | Prymnesiophytes, Diatoms, Dinophytes | Highly effective antioxidant systems, well-growing species |
| Halophilic species [144] | High salinity level, osmotic stress regulation | Chlorophytes and cyanophytes | Low growth capacity, costly culture management Molecules of interest |
3.3. Functional Diversity: Uptake of Nutritional Resources
| Name | Microalgae | Specificities |
|---|---|---|
| f/2 medium [148,149] | Coastal microalgae, diatoms | Enriched medium |
| K medium [146,150] | Oceanic microalgae | Trace metals |
| Pro99 [151] | Prochlorococcus spp. and some picoeukaryotes | High ammonia concentrations, No vitamin requirement |
| MNK medium [152] | Oceanic coccolithophores | Enriched medium |
3.4. Functional Diversity: Functional Traits versus Environmental Variables
4. Enhancing Production and Growth: “Photosynthetic Regulation Biotechnology” and Genetic Modifications
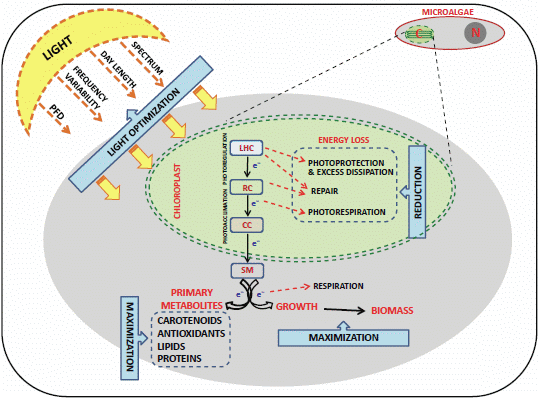
4.1. Light Control and the “Photosynthetic Regulation Biotechnology”

4.2. Genetic Transformations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Public Algaebase: Listing the World’s Algae. Available online: http://www.algaebase.org (accessed on 10 October 2013).
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef]
- Tomas, C.R. Identifying Marine Phytoplankton; Academic Press: San Diego, CA, USA, 1997; p. 858. [Google Scholar]
- Lebeau, T.; Robert, J.M. Diatom cultivation and biotechnologically relevant products. Part II: Current and putative products. Appl. Microbiol. Biotechnol. 2003, 60, 624–632. [Google Scholar] [CrossRef]
- Chu, W.-L. Biotechnological applications of microalgae. IeJSME 2012, 6, S24–S37. [Google Scholar]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Cadoret, J.-P.; Garnier, M.; Saint-Jean, B. Microalgae, Functional Genomics and Biotechnology. Adv. Bot. Res. 2012, 64, 285–341. [Google Scholar] [CrossRef]
- Zeiler, K.G.; Heacox, D.A.; Toon, S.T.; Kadam, K.L.; Brown, L.M. The use of microalgae for assimilation and utilization of carbon-dioxide from fossil fuel-fired power plant flue gas. Energy Convers. Manag. 1995, 36, 707–712. [Google Scholar] [CrossRef]
- Wilde, E.W.; Benemann, J.R. Bioremoval of heavy-metals by the use of microalgae. Biotechnol. Adv. 1993, 11, 781–812. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Becker, W. Microalgae in Human and Animal Nutrition. In Handbook of Microalgal Culture; Richmond, A., Ed.; Blackwell: Oxford, UK, 2004; pp. 312–351. [Google Scholar]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesel production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Sunda, W.; Kieber, D.J.; Kiene, R.P.; Huntsman, S. An antioxidant function for DMSP and DMS in marine algae. Nature 2002, 418, 317–320. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Munir, N.; Sharif, N.; Naz, S.; Manzoor, F. Algae: A potent antioxidant source. Sky J. Microbiol. Res. 2013, 1, 22–31. [Google Scholar]
- Dubinsky, Z.; Stambler, N. Photoacclimation processes in phytoplankton: Mechanisms, consequences, and applications. Aquat. Microb. Ecol. 2009, 56, 163–176. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically-active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Microalgal biomass production: Challenges and realities. Photosynth. Res. 2010, 106, 135–144. [Google Scholar] [CrossRef]
- Scalco, E.; Brunet, C.; Marino, F.; Rossi, R.; Soprano, V.; Zingone, A.; Montresor, M. Growth and toxicity responses of Mediterranean Ostreopsis cf. ovata to seasonal irradiance and temperature conditions. Harmful Algae 2012, 17, 25–34. [Google Scholar] [CrossRef]
- Meseck, S.L.; Smith, B.C.; Wikfors, G.H.; Alix, J.H.; Kapareiko, D. Nutrient interactions between phytoplankton and bacterioplankton under different carbon dioxide regimes. J. Appl. Phycol. 2007, 19, 229–237. [Google Scholar] [CrossRef]
- Brunet, C.; Johnsen, G.; Lavaud, J.; Roy, S. Pigments and Photoacclimation Processes. In Phytoplankton Pigments, Characterization, Chemotaxonomy and Applications in Oceanography; Roy, S., Johnsen, G., Llewellyn, C., Egeland, E.S., Eds.; SCOR-UNESCO Publishing, Cambridge University Press: Cambridge, UK, 2011; Volume 2, pp. 445–471. [Google Scholar]
- Dimier, C.; Saviello, G.; Tramontano, F.; Brunet, C. Comparative ecophysiology of the xanthophyll cycle in six marine phytoplanktonic species. Protist 2009, 160, 397–411. [Google Scholar] [CrossRef]
- Giovagnetti, V.; Cataldo, M.L.; Conversano, F.; Brunet, C. Growth and photophysiological responses of two picoplanktonic Minutocellus species, strains RCC967 and RCC703 (Bacillariophyceae). Eur. J. Phycol. 2012, 47, 408–420. [Google Scholar]
- Lebeau, T.; Robert, J. Biotechnology of immobilized micro algae: A Culture Technique for the Future? In Algal Cultures, Analogues of Blooms and Applications; Rao, S., Ed.; Science Publisher Inc.: New Hampshire, NH, USA, 2006; Volume 2, pp. 801–837. [Google Scholar]
- Hallmann, A. Algal transgenics and biotechnology. Transgenic Plant. J. 2007, 1, 81–98. [Google Scholar]
- Torres, M.A.; Barros, M.P.; Campos, S.C.G.; Pinto, E.; Rajamani, S.; Sayre, R.T.; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: A review. Ecotoxicol. Environ. Saf. 2008, 71, 1–15. [Google Scholar] [CrossRef]
- Montone, R.C.; Taniguchi, S.; Weber, R.R. Polychlorinated biphenyls in marine sediments of Admiralty Bay, King George Island, Antarctica. Mar. Pollut. Bull. 2001, 42, 611–614. [Google Scholar] [CrossRef]
- Leitão, M.A.d.S.; Cardozo, K.H.M.; Pinto, E.; Colepicolo, P. PCB-Induced oxidative stress in the unicellular marine dinoflagellate Lingulodinium polyedrum. Arch. Environ. Contam. Toxicol. 2003, 45, 59–65. [Google Scholar] [CrossRef]
- Gerofke, A.; Kömp, P.; McLachlan, M.S. Bioconcentration of persistent organic pollutants in four species of marine phytoplankton. Environ. Toxicol. Chem. 2005, 24, 2908–2917. [Google Scholar] [CrossRef]
- Aksmann, A.; Tukaj, Z. The effect of anthracene and phenanthrene on the growth, photosynthesis, and SOD activity of the green alga Scenedesmus armatus depends on the PAR Irradiance and CO2 level. Arch. Environ. Contam. Toxicol. 2004, 47, 177–184. [Google Scholar]
- Djomo, J.E.; Dauta, A.; Ferrier, V.; Narbonne, J.F.; Monkiedje, A.; Njine, T.; Garrigues, P. Toxic effects of some major polyaromatic hydrocarbons found in crude oil and aquatic sediments on Scenedesmus subspicatus. Water Res. 2004, 38, 1817–1821. [Google Scholar] [CrossRef]
- Lei, A.-P.; Hu, Z.-L.; Wong, Y.-S.; Tam, N.F.-Y. Removal of fluoranthene and pyrene by different microalgal species. Bioresour. Technol. 2007, 98, 273–280. [Google Scholar] [CrossRef]
- Doick, K.J.; Klingelmann, E.; Burauel, P.; Jones, K.C.; Semple, K.T. Long-term fate of polychlorinated biphenyls and polycyclic aromatic hydrocarbons in an agricultural soil. Environ. Sci. Technol. 2005, 39, 3663–3670. [Google Scholar] [CrossRef]
- Borja, J.; Taleon, D.M.; Auresenia, J.; Gallardo, S. Polychlorinated biphenyls and their biodegradation. Process Biochem. 2005, 40, 1999–2013. [Google Scholar] [CrossRef]
- Geoffroy, L.; Teisseire, H.; Couderchet, M.; Vernet, G. Effect of oxyfluorfen and diuron alone and in mixture on antioxidative enzymes of Scenedesmus obliquus. Pestic. Biochem. Physiol. 2002, 72, 178–185. [Google Scholar] [CrossRef]
- Nyström, B.; Becker-Van Slooten, K.; Bérard, A.; Grandjean, D.; Druart, J.-C.; Leboulanger, C. Toxic effects of Irgarol 1051 on phytoplankton and macrophytes in Lake Geneva. Water Res. 2002, 36, 2020–2028. [Google Scholar] [CrossRef]
- Geoffroy, L.; Dewez, D.; Vernet, G.; Popovic, R. Oxyfluorfen toxic effect on S. obliquus evaluated by different photosynthetic and enzymatic biomarkers. Arch. Environ. Contam. Toxicol. 2003, 45, 445–452. [Google Scholar] [CrossRef]
- Ma, J.; Chen, J. How to accurately assay the algal toxicity of pesticides with low water solubility. Environ. Pollut. 2005, 136, 267–273. [Google Scholar] [CrossRef]
- Cai, X.; Liu, W.; Jin, M.; Lin, K. Relation of diclofop-methyl toxicity and degradation in algae cultures. Environ. Toxicol. Chem. 2007, 26, 970–975. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Conti, M.E.; Cecchetti, G. A biomonitoring study: Trace metals in algae and molluscs from Tyrrhenian coastal areas. Environ. Res. 2003, 93, 99–112. [Google Scholar] [CrossRef]
- Pinto, E.; Sigaud-kutner, T.C.S.; Leitão, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Nishikawa, K.; Yamakoshi, Y.; Uemura, I.; Tominaga, N. Ultrastructural changes in Chlamydomonas acidophila (Chlorophyta) induced by heavy metals and polyphosphate metabolism. FEMS Microbiol. Ecol. 2003, 44, 253–259. [Google Scholar] [CrossRef]
- Mallick, N. Copper-induced oxidative stress in the chlorophycean microalga Chlorella vulgaris: Response of the antioxidant system. J. Plant Physiol. 2004, 161, 591–597. [Google Scholar] [CrossRef]
- Perales-Vela, H.V.; Peña-Castro, J.M.; Cañizares-Villanueva, R.O. Heavy metal detoxification in eukaryotic microalgae. Chemosphere 2006, 64, 1–10. [Google Scholar] [CrossRef]
- Tripathi, B.N.; Mehta, S.K.; Amar, A.; Gaur, J.P. Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu2+ and Zn2+. Chemosphere 2006, 62, 538–544. [Google Scholar] [CrossRef]
- Schroda, M.; Vallon, O.; Wollman, F.A.; Beck, C.F. A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell 1999, 11, 1165–1178. [Google Scholar]
- Ireland, H.E.; Harding, S.J.; Bonwick, G.A.; Jones, M.; Smith, C.J.; Williams, J.H.H. Evaluation of heat shock protein 70 as a biomarker of environmental stress in Fucus serratus and Lemna minor. Biomarkers 2004, 9, 139–155. [Google Scholar] [CrossRef]
- Singer, A.C.; Thompson, I.P.; Bailey, M.J. The tritrophic trinity: A source of pollutant-degrading enzymes and its implications for phytoremediation. Curr. Opin. Microbiol. 2004, 7, 239–244. [Google Scholar] [CrossRef]
- Lewis, S.; Donkin, M.E.; Depledge, M.H. Hsp70 expression in Enteromorpha intestinalis (Chlorophyta) exposed to environmental stressors. Aquat. Toxicol. 2001, 51, 277–291. [Google Scholar] [CrossRef]
- Spijkerman, E.; Barua, D.; Gerloff-Elias, A.; Kern, J.; Gaedke, U.; Heckathorn, S. Stress responses and metal tolerance of Chlamydomonas acidophila in metal-enriched lake water and artificial medium. Extremophiles 2007, 11, 551–562. [Google Scholar] [CrossRef]
- Andrade, L.R.; Farina, M.; Amado Filho, G.M. Effects of copper on Enteromorpha flexuosa (Chlorophyta) in vitro. Ecotoxicol. Environ. Saf. 2004, 58, 117–125. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Claxton, R.; Das, K.C. Biomass and bioenergy production potential of microalgae consortium in open and closed bioreactors using untreated carpet industry effluent as growth medium. Bioresour. Technol. 2010, 101, 6751–6760. [Google Scholar] [CrossRef]
- Sayre, R. Microalgae: The potential for carbon capture. BioScience 2010, 60, 722–727. [Google Scholar] [CrossRef]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the US Department of Energy’s Aquatic Species Program.: Biodiesel from Algae; National Renewable Energy Laboratory Golden: Golden, CO, USA, 1998; Volume 328, p. 294. [Google Scholar]
- Tsukahara, K.; Sawayama, S. Liquid fuel production using microalgae. J. Jpn. Pet. Inst. 2005, 48, 251–259. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biofuels from microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energ. Rev. 2010, 14, 217–232. [Google Scholar]
- Renaud, S.M.; Thinh, L.V.; Parry, D.L. The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture. Aquaculture 1999, 170, 147–159. [Google Scholar] [CrossRef]
- Pratoomyot, J.; Srivilas, P.; Noiraksar, T. Fatty acids composition of 10 microalgal species. Songklanakarin J. Sci. Technol. 2005, 27, 1179–1187. [Google Scholar]
- Aslan, S.; Kapdan, I.K. Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol. Eng. 2006, 28, 64–70. [Google Scholar] [CrossRef]
- Jin, E.; Polle, J.E.W.; Lee, H.K.; Hyun, S.M.; Chang, M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J. Microbiol. Biotechnol. 2003, 13, 165–174. [Google Scholar]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-K. Commercial production of microalgae in the Asia-Pacific rim. J. Appl. Phycol. 1997, 9, 403–411. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Microalgal reactors: A review of enclosed system designs and performances. Biotechnol. Prog. 2006, 22, 1490–1506. [Google Scholar] [CrossRef]
- Cornet, J.F. Le technoscope: Les photobioréacteurs. Biofutur 1998, 176, 1–10. [Google Scholar]
- Soletto, D.; Binaghi, L.; Lodi, A.; Carvalho, J.C.M.; Converti, A. Batch and fed-batch cultivations of Spirulina platensis using ammonium sulphate and urea as nitrogen sources. Aquaculture 2005, 243, 217–224. [Google Scholar] [CrossRef]
- Yamaguchi, K. Recent advances in microalgal bioscience in Japan, with special reference to utilization of biomass and metabolites: A review. J. Appl. Phycol. 1996, 8, 487–502. [Google Scholar] [CrossRef]
- Liang, S.Z.; Liu, X.M.; Chen, F.; Chen, Z.J. Current microalgal health food R & D activities in China. Hydrobiologia 2004, 512, 45–48. [Google Scholar] [CrossRef]
- Apt, K.E.; Behrens, P.W. Commercial developments in microalgal biotechnology. J. Phycol. 1999, 35, 215–226. [Google Scholar] [CrossRef]
- Iwamoto, K.; Shiraiwa, Y. Salt-regulated mannitol metabolism in algae. Mar. Biotechnol. 2005, 7, 407–415. [Google Scholar] [CrossRef]
- Metting, F.B. Biodiversity and application of microalgae. J. Ind. Microbiol. Biotechnol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodriguez, H.; Moreno, J.; Vargas, M.A.; Guerrero, M.G. Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J. Biotechnol. 2001, 85, 289–295. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Yamaguchi, K.; Nishio, N.; Nagai, S. Growth and astaxanthin formation of Haematococcus pluvialis in heterotrophic and mixotrophic conditions. J. Ferment. Bioeng. 1992, 74, 17–20. [Google Scholar] [CrossRef]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Radmer, R.J. Algal diversity and commercial algal products. BioScience 1996, 46, 263–270. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.d.O.; Danesi, E.D.G.; de Carvalho, J.C.M.; Sato, S. Chlorophyll production from Spirulina platensis: Cultivation with urea addition by fed-batch process. Bioresour. Technol. 2004, 92, 133–141. [Google Scholar] [CrossRef]
- Jensen, G.S.; Ginsberg, D.I.; Drapeau, C. Blue-green algae as an immuno-enhancer and biomodulator. J. Am. Nutraceut. Assoc. 2001, 3, 24–30. [Google Scholar]
- Benedetti, S.; Benvenuti, F.; Pagliarani, S.; Francogli, S.; Scoglio, S.; Canestrari, F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004, 75, 2353–2362. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae for aquaculture: Opportunities and constraints. J. Appl. Phycol. 1997, 9, 393–401. [Google Scholar] [CrossRef]
- Muller-Feuga, A. The role of microalgae in aquaculture: Situation and trends. J. Appl. Phycol. 2000, 12, 527–534. [Google Scholar] [CrossRef]
- Brown, M.R.; Mular, M.; Miller, I.; Farmer, C.; Trenerry, C. The vitamin content of microalgae used in aquaculture. J. Appl. Phycol. 1999, 11, 247–255. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Stolz, P.; Obermayer, B. Manufacturing microalgae for skin care. Cosmet. Toilet. 2005, 120, 99–106. [Google Scholar]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Pushparaj, B.; Pelosi, E.; Carlozzi, P.; Torzillo, G. Yield and biochemical-composition of a marine cyanobacterium (Nodularia sp.) in outdoor culture. Aquat. Microb. Ecol. 1995, 9, 13–16. [Google Scholar] [CrossRef]
- Vonshak, A.; Chanawongse, L.; Bunnag, B.; Tanticharoen, M. Light acclimation and photoinhibition in three Spirulina platensis (cyanobacteria) isolates. J. Appl. Phycol. 1996, 8, 35–40. [Google Scholar] [CrossRef]
- Torzillo, G.; Bernardini, P.; Masojidek, J. On-line monitoring of chlorophyll fluorescence to assess the extent of photoinhibition of photosynthesis induced by high oxygen concentration and low temperature and its effect on the productivity of outdoor cultures of Spirulina platensis (Cyanobacteria). J. Phycol. 1998, 34, 504–510. [Google Scholar] [CrossRef]
- Molina, E.; Fernandez, J.; Acien, F.G.; Chisti, Y. Tubular photobioreactor design for algal cultures. J. Biotechnol. 2001, 92, 113–131. [Google Scholar] [CrossRef]
- Roy, L.A.; Davis, D.A.; Saoud, I.P. Effects of lecithin and cholesterol supplementation to practical diets for Litopenaeus vannamei reared in low salinity waters. Aquaculture 2006, 257, 446–452. [Google Scholar] [CrossRef]
- Ugwu, C.U.; Aoyagi, H.; Uchiyama, H. Influence of irradiance, dissolved oxygen concentration, and temperature on the growth of Chlorella sorokiniana. Photosynthetica 2007, 45, 309–311. [Google Scholar] [CrossRef]
- Masojídek, J.; Vonshak, A.; Torzillo, G. Chlorophyll Fluorescence Applications in Microalgal Mass Cultures. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Suggett, D.J., Prášil, O., Borowitzka, M.A., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 4, pp. 277–292. [Google Scholar]
- Giovagnetti, V.; Brunet, C.; Conversano, F.; Tramontano, F.; Obernosterer, I.; Ridame, C.; Guieu, C. Assessing the role of dust deposition on phytoplankton ecophysiology and succession in a low-nutrient low-chlorophyll ecosystem: A mesocosm experiment in the Mediterranean Sea. BioGeosciences 2013, 10, 2973–2991. [Google Scholar] [CrossRef] [Green Version]
- Pulz, O. Photobioreactors: Production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 2001, 57, 287–293. [Google Scholar] [CrossRef]
- Xu, L.; Weathers, P.J.; Xiong, X.-R.; Liu, C.-Z. Microalgal bioreactors: Challenges and opportunities. Eng. Life Sci. 2009, 9, 178–189. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A. Trait-based Community Ecology of Phytoplankton. Ann. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef]
- Kooistra, W.H.C.F.; Gersonde, R.; Medlin, L.K.; Mann, D.G. The Origin and Evolution of the Diatoms: Their Adaptation to a Planktonic Existence. In Evolution of Primary Producers in the Sea; Paul, G.F., Andrew, H.K., Eds.; Academic Press: Burlington, Canada, 2007; pp. 207–249. [Google Scholar]
- Mann, D.G.; Droop, S.J.M. Biodiversity, biogeography and conservation of diatoms. Hydrobiologia 1996, 336, 19–32. [Google Scholar] [CrossRef]
- Amato, A.; Kooistra, W.H.C.F.; Ghiron, J.H.L.; Mann, D.G.; Proschold, T.; Montresor, M. Reproductive isolation among sympatric cryptic species in marine diatoms. Protist 2007, 158, 193–207. [Google Scholar]
- Amato, A.; Montresor, M. Morphology, phylogeny, and sexual cycle of Pseudo-nitzschia mannii sp. nov. (Bacillariophyceae): A pseudo-cryptic species within the P. pseudodelicatissima complex. Phycologia 2008, 47, 487–497. [Google Scholar] [CrossRef]
- Medlin, L.K.; Kooistra, W.H.C.F. Methods to estimate the diversity in the marine photosynthetic protist community with illustrations from case studies: A review. Diversity 2010, 2, 973–1014. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Katz, M.E.; Knoll, A.H.; Quigg, A.; Raven, J.A.; Schofield, O.; Taylor, F.J.R. The evolution of modern eukaryotic phytoplankton. Science 2004, 305, 354–360. [Google Scholar] [CrossRef]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef]
- Tirichine, L.; Bowler, C. Decoding algal genomes: Tracing back the history of photosynthetic life on Earth. Plant. J. 2011, 66, 45–57. [Google Scholar] [CrossRef]
- Reusch, T.B.H.; Boyd, P.W. Experimental evolution meets marine phytoplankton. Evolution 2013, 67, 1849–1859. [Google Scholar] [CrossRef]
- Groben, R.; John, U.; Eller, G.; Lange, M.; Medlin, L.K. Using fluorescently-labelled rRNA probes for hierarchical estimation of phytoplankton diversity—A mini-review. Nova Hedwig. 2004, 79, 313–320. [Google Scholar] [CrossRef]
- Orsini, L.; Procaccini, G.; Sarno, D.; Montresor, M. Multiple rDNA ITS-types within the diatom Pseudo-nitzschia delicatissima (Bacillariophyceae) and their relative abundances across a spring bloom in the Gulf of Naples. Mar. Ecol. Prog. Ser. 2004, 271, 87–98. [Google Scholar] [CrossRef]
- Massana, R.; Castresana, J.; Balagué, V.; Guillou, L.; Romari, K.; Groisillier, A.; Valentin, K.; Pedrós-Alió, C. Phylogenetic and ecological analysis of novel marine stramenopiles. Appl. Environ. Microbiol. 2004, 70, 3528–3534. [Google Scholar] [CrossRef]
- Sarno, D.; Kooistra, W.H.C.F.; Medlin, L.K.; Percopo, I.; Zingone, A. Diversity in the genus Skeletonema (bacillariophyceae). (ii) An assessment of the taxonomy of S. costatum like species with the description of four new species. J. Phycol. 2005, 41, 151–176. [Google Scholar] [CrossRef] [Green Version]
- Smayda, T.J. The suspension and sinking of phytoplankton in the sea. Oceanogr. Mar. Biol. Annu. Rev. 1970, 8, 353–414. [Google Scholar]
- Pickett-Heaps, J.D. Cell division and moprhogenesis of the centric diatom Chaetoceros decipiens (bacillariophyceae) I. Living cells. J. Phycol. 1998, 34, 989–994. [Google Scholar]
- Yokota, K.; Sterner, R.W. Trade-offs limiting the evolution of coloniality: Ecological displacement rates used to measure small costs. Proc. Biol. Sci. 2011, 278, 458–463. [Google Scholar] [CrossRef]
- Rousseau, V.; Chrétiennot-Dinet, M.-J.; Jacobsen, A.; Verity, P.; Whipple, S. The life cycle of Phaeocystis: State of knowledge and presumptive role in ecology. Biogeochemistry 2007, 83, 29–47. [Google Scholar] [CrossRef]
- Young, A.M.; Karp-Boss, L.; Jumars, P.A.; Landis, E.N. Quantifying diatom aspirations: Mechanical properties of chain-forming species. Limnol. Oceanogr. 2012, 57, 1789–1801. [Google Scholar] [CrossRef]
- Smetacek, V.; Assmy, P.; Henjes, J. The role of grazing in structuring Southern Ocean pelagic ecosystems and biogeochemical cycles. Antarct. Sci. 2004, 16, 541–558. [Google Scholar] [CrossRef]
- Bergkvist, J.; Thor, P.; Jakobsen, H.H.; Wangberg, S.-A.; Selander, E. Grazer-induced chain length plasticity reduces grazing risk in a marine diatom. Limnol. Oceanogr. 2012, 57, 318–324. [Google Scholar]
- Karp-Boss, L.; Boss, E.; Jumars, P.A. Nutrient fluxes to planktonic osmotrophs in the presence of fluid motion. Oceanogr. Mar. Biol. Annu. Rev. 1996, 34, 71–107. [Google Scholar]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef]
- Not, F.; Massana, R.; Latasa, M.; Marie, D.; Colson, C.; Eikrem, W.; Pedros-Alio, C.; Vaulot, D.; Simon, N. Late summer community composition and abundance of photosynthetic picoeukaryotes in Norwegian and Barents Seas. Limnol. Oceanogr. 2005, 50, 1677–1686. [Google Scholar] [CrossRef]
- Worden, A.Z.; Not, F. Ecology and Diversity of Picoeukaryotes. In Microbial Ecology of the Oceans; Kirchman, D.L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 159–205. [Google Scholar]
- Raven, J.A. The twelfth Tansley Lecture. Small is beautiful: The picophytoplankton. Funct. Ecol. 1998, 12, 503–513. [Google Scholar] [CrossRef]
- Raven, J.A.; Finkel, Z.V.; Irwin, A.J. Picophytoplankton: Bottom-up and top-down controls on ecology and evolution. Vie Milieu Life Environ. 2005, 55, 209–215. [Google Scholar]
- Palenik, B.; Grimwood, J.; Aerts, A.; Rouze, P.; Salamov, A.; Putnam, N.; Dupont, C.; Jorgensen, R.; Derelle, E.; Rombauts, S.; et al. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc. Natl. Acad. Sci. USA 2007, 104, 7705–7710. [Google Scholar] [CrossRef]
- Six, C.; Finkel, Z.V.; Rodriguez, F.; Marie, D.; Partensky, F.; Campbell, D.A. Contrasting photoacclimation costs in ecotypes of the marine eukaryotic picoplankter Ostreococcus. Limnol. Oceanogr. 2008, 53, 255–265. [Google Scholar] [CrossRef]
- Six, C.; Sherrard, R.; Lionard, M.; Roy, S.; Campbell, D.A. Photosystem II and pigment dynamics among ecotypes of the green alga Ostreococcus. Plant Physiol. 2009, 151, 379–390. [Google Scholar] [CrossRef]
- Cox, E.R. Phytoflagellates; Elsevier North Holland: New York, NY, USA, 1980. [Google Scholar]
- MacIntyre, H.; Geider, R.; Miller, D. Microphytobenthos: The ecological role of the “secret garden” of unvegetated, shallow-water marine habitats. I. Distribution, abundance and primary production. Estuaries 1996, 19, 186–201. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Chepurnov, V.A.; Mann, D.G.; Sabbe, K.; Vyverman, W. Experimental Studies an Sexual Reproduction in Diatoms. In International Review of Cytology—A Survey of Cell Biology; Jeon, K.W., Ed.; Elsevier Academic Press: San Diego, USA, 2004; Volume 237, pp. 91–154. [Google Scholar]
- Berman-Frank, I.; Quigg, A.; Finkel, Z.V.; Irwin, A.J.; Haramaty, L. Nitrogen-fixation strategies and Fe requirements in cyanobacteria. Limnol. Oceanogr. 2007, 52, 2260–2269. [Google Scholar] [CrossRef]
- Flynn, K.J.; Stoecker, D.K.; Mitra, A.; Raven, J.A.; Glibert, P.M.; Hansen, P.J.; Granéli, E.; Burkholder, J.M. Misuse of the phytoplankton–zooplankton dichotomy: The need to assign organisms as mixotrophs within plankton functional types. J. Plankton Res. 2013, 35, 3–11. [Google Scholar] [CrossRef]
- Wilken, S.; Huisman, J.; Naus-Wiezer, S.; Donk, E. Mixotrophic organisms become more heterotrophic with rising temperature. Ecol. Lett. 2013, 16, 225–233. [Google Scholar] [CrossRef]
- Stoecker, D.K. Conceptual models of mixotrophy in planktonic protists and some ecological and evolutionary implications. Eur. J. Protistol. 1998, 34, 281–290. [Google Scholar] [CrossRef]
- Falkowski, P.G. Light-shade adaptation and vertical mixing of marine-phytoplankton—A comparative field-study. J. Mar. Res. 1983, 41, 215–237. [Google Scholar] [CrossRef]
- Marchetti, A.; Maldonado, M.T.; Lane, E.S.; Harrison, P.J. Iron requirements of the pennate diatom Pseudo-nitzschia: Comparison of oceanic (high-nitrate, low-chlorophyll waters) and coastal species. Limnol. Oceanogr. 2006, 51, 2092–2101. [Google Scholar] [CrossRef]
- Hare, C.E.; Leblanc, K.; DiTullio, G.R.; Kudela, R.M.; Zhang, Y.; Lee, P.A.; Riseman, S.; Hutchins, D.A. Consequences of increased temperature and CO2 for phytoplankton community structure in the Bering Sea. Mar. Ecol. Prog. Ser. 2007, 352, 9–16. [Google Scholar] [CrossRef]
- Toseland, A.; Daines, S.J.; Clark, J.R.; Kirkham, A.; Strauss, J.; Uhlig, C.; Lenton, T.M.; Valentin, K.; Pearson, G.A.; Moulton, V. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim. Chang. 2013, 3, 979–984. [Google Scholar] [CrossRef]
- Takano, H.; Furuune, H.; Burgess, J.G.; Manabe, E.; Hirano, M.; Okazaki, M.; Matsunaga, T. Production of ultrafine calcite particles by coccolithophorid algae grown in a biosolar reactor supplied with sunlight. Appl. Biochem. Biotechnol. 1993, 39, 159–167. [Google Scholar]
- DasSarma, P.; Coker, J.A.; Huse, V.; DasSarma, S. Halophiles, Industrial Applications. In Encyclopedia of Industrial Biotechnology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 1–43. [Google Scholar]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar]
- The National Center for Marine Algae and Microbiota (NCMA). Culturing Diversity. Available online: https://ncma.bigelow.org (accessed on 10 October 2013).
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Keller, M.D.; Guillard, R.R.L. Factors Significant to Marine Diatom Culture. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 113–116. [Google Scholar]
- Moore, L.R.; Coe, A.; Zinser, E.R.; Saito, M.A.; Sullivan, M.B.; Lindell, D.; Frois-Moniz, K.; Waterbury, J.; Chisholm, S.W. Culturing the marine cyanobacterium Prochlorococcus. Limnol. Oceanogr. Methods 2007, 5, 353–362. [Google Scholar] [CrossRef]
- Noel, M.H.; Kawachi, M.; Inouye, I. Induced dimorphic life cycle of a coccolithophorid, Calyptrosphaera sphaeroidea (Prymnesiophyceae, Haptophyta). J. Phycol. 2004, 40, 112–129. [Google Scholar] [CrossRef]
- Andersen, T.; Schartau, A.; Paasche, E. Quantifying external and internal nitrogen and phosphorus pools, as well as nitrogen and phosphorus supplied through remineralization, in coastal marine plankton by means of a dilution technique. Mar. Ecol. Prog. Ser. 1991, 69, 67–80. [Google Scholar] [CrossRef]
- Irwin, A.J.; Finkel, Z.V.; Schofield, O.M.E.; Falkowski, P.G. Scaling-up from nutrient physiology to the size-structure of phytoplankton communities. J. Plankton Res. 2006, 28, 459–471. [Google Scholar] [CrossRef]
- Verdy, A.; Follows, M.; Flierl, G. Optimal phytoplankton cell size in an allometric model. Mar. Ecol. Prog. Ser. 2009, 379, 1–12. [Google Scholar] [CrossRef]
- Shuter, B.J. Size dependence of phosphorus and nitrogen subsistence quotas in unicellular microorganisms. Limnol. Oceanogr. 1978, 23, 1248–1255. [Google Scholar] [CrossRef]
- Sommer, U. The paradox of the plankton: Fluctuations of phosphorus availability maintain diversity of phytoplankton in flow-through cultures. Limnol. Oceanogr. 1984, 29, 633–636. [Google Scholar] [CrossRef]
- Morel, F.M.M. Kinetics of nutrient-uptake and growth in phytoplankton. J. Phycol. 1987, 23, 137–150. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Bossard, P. Phytoplankton nutrient competition under dynamic light regimes. Limnol. Oceanogr. 2004, 49, 1457–1462. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Schofield, O.M.; Falkowski, P.G. The role of functional traits and trade-offs in structuring phytoplankton communities: Scaling from cellular to ecosystem level. Ecol. Lett. 2007, 10, 1170–1181. [Google Scholar] [CrossRef]
- Eppley, R.W.; Carlucci, A.F.; Holm-Hansen, O.; Kiefer, D.; McCarthy, J.J.; Venrick, E.; Williams, P.M. Phytoplankton growth and composition in shipboard cultures supplied with nitrate, ammonium, or urea as nitrogen source. Limnol. Oceanogr. 1971, 16, 741–751. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Manimaran, K.; Sampathkumar, P.; Rameshkumar, L. Growth and nutrient removal properties of the diatoms, Chaetoceros curvisetus and C. simplex under different nitrogen sources. Appl. Water Sci. 2013, 3, 49–55. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Barra, L.; Caruso, T.; Corsaro, L.; Piaz, F.D.; Graziani, G.; Corato, F.; Brunet, C. Light modulation on the biochemical properties of the diatom Skeletonema marinoi: Relevance of photosynthetic manipulation in the biotechnological field. 2014; Unpublished work. [Google Scholar]
- Allen, A.E.; Dupont, C.L.; Obornik, M.; Horak, A.; Nunes-Nesi, A.; McCrow, J.P.; Zheng, H.; Johnson, D.A.; Hu, H.; Fernie, A.R.; et al. Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature 2011, 473, 203–207. [Google Scholar] [CrossRef]
- Joy, K.W.; Hageman, R.H. The purification and properties of nitrite reductase from higher plants, and its dependence on ferredoxin. Biochem. J. 1966, 100, 263–273. [Google Scholar]
- Flynn, K.J.; Fasham, M.J.R.; Hipkin, C.R. Modelling the interactions between ammonium and nitrate uptake in marine phytoplankton. Phil. Trans. R Soc. B Biol. Sci. 1997, 352, 1625–1645. [Google Scholar] [CrossRef]
- Thomas, W.H.; Hastings, J.; Fujita, M. Ammonium input to the sea via large sewage outfalls-Part 2: Effects of ammonium on growth and photosynthesis of southern California phytoplankton cultures. Mar. Environ. Res. 1980, 3, 291–296. [Google Scholar] [CrossRef]
- Eppley, R.W.; Rogers, J.N.; McCarthy, J.J. Half-saturation constants for uptake of nitrate and ammonium by marine-phytoplankton. Limnol. Oceanogr. 1969, 14, 912–920. [Google Scholar] [CrossRef]
- Kamp, A.; de Beer, D.; Nitsch, J.L.; Lavik, G.; Stief, P. Diatoms respire nitrate to survive dark and anoxic conditions. Proc. Natl. Acad. Sci. USA 2011, 108, 5649–5654. [Google Scholar] [CrossRef]
- Kamp, A.; Stief, P.; Knappe, J.; de Beer, D. Response of the ubiquitous pelagic diatom Thalassiosira weissflogii to darkness and anoxia. PLoS One 2013, 8, e82605. [Google Scholar]
- Alonso, D.L.; Belarbi, E.-H.; Fernández-Sevilla, J.M.; Rodríguez-Ruiz, J.; Grima, E.M. Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochemistry 2000, 54, 461–471. [Google Scholar] [CrossRef]
- Reitan, K.I.; Rainuzzo, J.R.; Olsen, Y. Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J. Phycol. 1994, 30, 972–979. [Google Scholar]
- Lombardi, A.T.; Wangersky, P.J. Influence of phosphorus and silicon on lipia class production by the marine diatom Chaetoceros gracilis grown in turbidostat cage cultures. Mar. Ecol. Prog. Ser. 1991, 77, 39–47. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green-alga Haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar]
- Ben-Amotz, A.; Avron, M. On the factors which determine the massive bcarotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 1983, 72, 593–597. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Sukenik, A.; Herzig, R. Nitrogen limitation in Isochrysis galbana (haptophyceae). ii. Relative abundance of chloroplast proteins. J. Phycol. 1989, 25, 471–478. [Google Scholar] [CrossRef]
- Herrig, R.; Falkowski, P.G. Nirogen limitation in Isochrysis galbana (haptophyceae). I. Photosynthetic energy conversion and growth efficiencies. J. Phycol. 1989, 25, 462–471. [Google Scholar] [CrossRef]
- Geider, R.J.; La Roche, J.; Greene, R.M.; Olaizola, M. Response of the photosynthetic apparatus of Phaeodactylum tricornutum (bacillariophyceae) to nitrate, phosphate, or iron starvation. J. Phycol. 1993, 29, 755–766. [Google Scholar]
- Geider, R.J.; Macintyre, H.L.; Graziano, L.M.; McKay, R.M.L. Responses of the photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorus limitation. Eur. J. Phycol. 1998, 33, 315–332. [Google Scholar] [CrossRef]
- Lai, J.; Yu, Z.; Song, X.; Cao, X.; Han, X. Responses of the growth and biochemical composition of Prorocentrum donghaiense to different nitrogen and phosphorus concentrations. J. Exp. Mar. Biol. Ecol. 2011, 405, 6–17. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Cao, X.; Yu, Z. Responses of photosynthetic characters of Skeletonema costatum to different nutrient conditions. J. Plankton Res. 2013, 35, 165–176. [Google Scholar] [CrossRef]
- Beardall, J.; Johnston, A.; Raven, J. Environmental regulation of CO2 concentrating mechanisms in microalgae. Can. J. Bot. 1998, 76, 1010–1017. [Google Scholar]
- Martin, J.H. Iron, Liebig’s Law, and the greenhouse. Oceanography 1991, 4, 52–55. [Google Scholar] [CrossRef]
- Dixon, J.L. Macro and micro nutrient limitation of microbial productivity in oligotrophic subtropical Atlantic waters. Environ. Chem. 2008, 5, 135–142. [Google Scholar] [CrossRef]
- Strzepek, R.F.; Harrison, P.J. Photosynthetic architecture differs in coastal and oceanic diatoms. Nature 2004, 431, 689–692. [Google Scholar] [CrossRef]
- Raven, J.A. The iron and molybdenum use efficiencies of plant-growth with different energy, carbon and nitrogen-sources. New Phytol. 1988, 109, 279–287. [Google Scholar] [CrossRef]
- Maldonado, M.T.; Price, N.M. Influence of N substrate on Fe requirements of marine centric diatoms. Mar. Ecol. Prog. Ser. 1996, 141, 161–172. [Google Scholar] [CrossRef]
- Sunda, W.; Huntsman, S.A. Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 1997, 390, 389–392. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Reinfelder, J.R.; Roberts, S.B.; Chamberlain, C.P.; Lee, J.G.; Yee, D. Zinc and carbon co-limitation of marine-phytoplankton. Nature 1994, 369, 740–742. [Google Scholar] [CrossRef]
- Raven, J.A.; Evans, M.C.W.; Korb, R.E. The role of trace metals in photosynthetic electron transport in O2 evolving organisms. Photosynth. Res. 1999, 60, 111–149. [Google Scholar] [CrossRef]
- Price, N.M.; Morel, F.M.M. Co-limitation of phytoplankton growth by nickel and nitrogen. Limnol. Oceanogr. 1991, 36, 1071–1077. [Google Scholar] [CrossRef]
- Provasoli, L.; Carlucci, A.F. Vitamins and Growth Regulators. In Algal Physiology and Biochemistry; Stewart, W.D.P., Abbott, M.R, Eds.; Blackwell Scientific: Malden, MA, USA, 1974; pp. 741–787. [Google Scholar]
- Adolf, J.E.; Stoecker, D.K.; Harding, L.W. The balance of autotrophy and heterotrophy during mixotrophic growth of Karlodinium micrum (Dinophyceae). J. Plankton Res. 2006, 28, 737–751. [Google Scholar] [CrossRef]
- Hammer, A.C.; Pitchford, J.W. The role of mixotrophy in plankton bloom dynamics, and the consequences for productivity. ICES J. Mar. Sci. 2005, 62, 833–840. [Google Scholar] [CrossRef]
- Ward, B.A.; Dutkiewicz, S.; Barton, A.D.; Follows, M.J. Biophysical aspects of resource acquisition and competition in algal mixotrophs. Am. Nat. 2011, 178, 98–112. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Ma, C.; Zhao, L.; Ren, N.-Q. A new lipid-rich microalga Scenedesmus sp. strain R-16 isolated using Nile red staining: Effects of carbon and nitrogen sources and initial pH on the biomass and lipid production. Biotechnol. Biofuels 2013, 6, 143. [Google Scholar] [CrossRef]
- Ketchum, B.H. The development and restoration of deficiencies in the phosphorus and nitrogen composition of unicellular plants. J. Cell. Comp. Physiol. 1939, 12, 373–381. [Google Scholar] [CrossRef]
- Syrett, P.J. Nitrogen Assimilation. In Physiology and Biochemistry of Algae; Lewin, R.A., Ed.; Academic Press: New York, NY, USA, 1962; pp. 171–209. [Google Scholar]
- Terry, K.L.; Hirata, J.; Laws, E.A. Light-limited growth of two strains of the marine diatom Phaeodactylum tricornutum Bohlin: Chemical composition, carbon partitioning and the diel peridiocity of physiological processes. J. Exp. Mar. Biol. Ecol. 1983, 68, 209–227. [Google Scholar] [CrossRef]
- Curtis, P.J.; Megard, R.O. Interactions among irradiance, oxygen evolution and nitrite uptake by Chlamydomonas (chlorophyceae). J. Phycol. 1987, 23, 608–613. [Google Scholar] [CrossRef]
- Kerby, N.W.; Rowell, P.; Stewart, W.D.P. The Transport, Assimilation and Production of Nitrogenous Compounds by Cyanobacteria and Microalgae. In Algal and Cyanobacterial Biotechnology; Cresswell, R.C., Rees, T.A.V., Shah, N., Eds.; Longman Scientific & Technical: Essex, UK, 1989; pp. 50–90. [Google Scholar]
- Meseck, S.L.; Alix, J.H.; Wikfors, G.H. Photoperiod and light intensity effects on growth and utilization of nutrients by the aquaculture feed microalga, Tetraselmis chui (PLY429). Aquaculture 2005, 246, 393–404. [Google Scholar] [CrossRef]
- Fabregas, J.; Maseda, A.; Dominguez, A.; Otero, A. The cell composition of Nannochloropsis sp changes under different irradiances in semicontinuous culture. World J. Microbiol. Biotechnol. 2004, 20, 31–35. [Google Scholar] [CrossRef]
- Klyachko-Gurvich, G.L.; Tsoglin, L.N.; Doucha, J.; Kopetskii, J.; Shebalina, I.B.; Semenenko, V.E. Desaturation of fatty acids as an adaptive response to shifts in light intensity. Physiol. Plant. 1999, 107, 240–249. [Google Scholar] [CrossRef]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Franklin, L.A.; Osmond, C.B.; Larkum, A.W.D. Photoinhibition, UB-B and Algal Photosynthesis. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 351–384. [Google Scholar]
- Vonshak, A.; Torzillo, G. Environmental Stress Physiology. In Handbook of Microalgal Culture; Richmond, A., Ed.; Blackwell: Oxford, UK, 2004; pp. 57–82. [Google Scholar]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Lepetit, B.; Volke, D.; Gilbert, M.; Wilhelm, C.; Goss, R. Evidence for the existence of one antenna-associated, lipid-dissolved and two protein-bound pools of diadinoxanthin cycle pigments in diatoms. Plant Physiol. 2010, 154, 1905–1920. [Google Scholar] [CrossRef]
- Fábregas, J.; Dominguez, A.; Maseda, A.; Otero, A. Interactions between irradiance and nutrient availability during astaxanthin accumulation and degradation in Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2003, 61, 545–551. [Google Scholar] [CrossRef]
- García-Malea, M.C.; Acién, F.G.; Del Río, E.; Fernández, J.M.; Cerón, M.C.; Guerrero, M.G.; Molina-Grima, E. Production of astaxanthin by Haematococcus pluvialis: Taking the one-step system outdoors. Biotechnol. Bioeng. 2009, 102, 651–657. [Google Scholar] [CrossRef]
- Raven, J.A.; Geider, R.J. Temperature and algal growth. New Phytol. 1988, 110, 441–461. [Google Scholar] [CrossRef]
- Davison, I.R. Environmental-effects on algal photosynthesis—Temperature. J. Phycol. 1991, 27, 2–8. [Google Scholar] [CrossRef]
- Fogg, G.E. Algal Adaptation to Stress–Some General Remarks. In Algal Adaptation to Environmental Stresses: Physiological, Biochemical and Molecular Mechanisms; Rai, L.C., Gaur, J.P., Eds.; Springer-Verlag: New York, NY, USA, 2001; pp. 1–19. [Google Scholar]
- Eppley, R.W. Temperature and phytoplankton growth in the sea. Fish. Bull. 1972, 70, 1063–1085. [Google Scholar]
- Goldman, J.C.; Carpenter, E.J. A kinetic approach to the effect of temperature on algal growth. Limnol. Oceanogr. 1974, 19, 756–766. [Google Scholar] [CrossRef]
- Thompson, P.A.; Guo, M.; Harrison, P.J. Effects of variation in temperature. I. On the biochemical composition of eight species of marine phytoplankton. J. Phycol. 1992, 28, 481–488. [Google Scholar]
- Thompson, P.A.; Guo, M.; Harrison, P.J.; Whyte, J.N.C. Effects of variation in temperature.II. On the fatty acid composition of eight species of marine phytoplankton. J. Phycol. 1992, 28, 488–497. [Google Scholar]
- Gao, Y.; Smith, G.J.; Alberte, R.S. Temperature dependence of nitrate reductase activity in marine phytoplankton: Biochemical analysis and ecological, implications. J. Phycol. 2000, 36, 304–313. [Google Scholar]
- Lomas, M.W.; Glibert, P.M. Interactions between NH4+ and NO3− uptake and assimilation: Comparison of diatoms and dinoflagellates at several growth temperatures. Mar. Biol. 1999, 133, 541–551. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Temperature regulation of nitrate uptake: A novel hypothesis about nitrate uptake and reduction in cool-water diatoms. Limnol. Oceanogr. 1999, 44, 556–572. [Google Scholar] [CrossRef]
- Payer, H.D.; Chiemvichak, Y.; Hosakul, K.; Kongpanichkul, C.; Kraidej, L.; Nguitragul, M.; Reungmanipytoon, S.; Buri, P. Temperature as an important climatic factor during mass production of microscopic algae. In Algae Biomass; Shelef, G., Soeder, C.J., Eds.; Elsevier/North- Holland Biomedical Press: Amsterdam, The Netherlands, 1980; pp. 389–399. [Google Scholar]
- De Oliveira, M.; Monteiro, M.; Robbs, P.; Leite, S. Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquac. Int. 1999, 7, 261–275. [Google Scholar] [CrossRef]
- Marre, E. Temperature. In Physiology and Biochemistry of Algae; Lewin, R.A., Ed.; Academic Press: New York, NY, USA, 1962; pp. 541–550. [Google Scholar]
- Teoh, M.L.; Chu, W.L.; Marchant, H.; Phang, S.M. Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J. Appl. Phycol. 2004, 16, 421–430. [Google Scholar] [CrossRef]
- Nelson, J.R.; Guarda, S.; Cowell, L.E.; Heffernan, P.B. Evaluation of microalgal clones for mass culture in a subtropical greenhouse bivalve hatchery: Growth-rates and biochemical composition at 30 °C. Aquaculture 1992, 106, 357–377. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry and Biotechnology; Taylor and Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Thomas, W.H.; Tornabene, T.G.; Weissman, J.C. Screening for Lipid Yielding Microalgae: Activities for 1983; SERI/STR-231-2207 Solar Energy Research Institute: Golden, Colorado, 1984. [Google Scholar]
- Kirst, G.O. Coordination of ionic relations and mannitol concentrations in the euryhaline unicellular alga, Platymonas subcordiformis (Hazen) after osmotic shocks. Planta 1977, 135, 69–75. [Google Scholar] [CrossRef]
- Strizh, I.; Popova, L.; Balnokin, Y.V. Physiological aspects of adaptation of the marine microalga Tetraselmis (Platymonas) viridis to various medium salinity. Russ. J. Plant Physiol. 2004, 51, 176–182. [Google Scholar] [CrossRef]
- Borowitzka, L.J.; Brown, A.D. The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch. Mikrobiol. 1974, 96, 37–52. [Google Scholar] [CrossRef]
- Kirst, G.O. Turgor Pressure Regulation in Marine Macroalgae. In Experimental Phycology; Lobban, C.S., Chapman, D.J., Kremer, B.P., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 203–209. [Google Scholar]
- Reed, R.H.; Stewart, W.D.P. The Responses of Cyanobacteria to Salt Stress. In Biochemistry of the Algae and Cyanobacteria; Rogers, L.J., Gallon, J.R., Eds.; Oxford University Press: London, UK, 1988; pp. 217–231. [Google Scholar]
- Kirst, G.O. Salinity tolerance of eukaryotic marine-algae. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 21–53. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Tornabene, T.G.; Thomas, W.H. Chemical profile of selected species of microalgae with emphasis on lipids. J. Phycol. 1985, 21, 72–81. [Google Scholar] [CrossRef]
- Renaud, S.M.; Parry, D.L. Microalgae for use in tropical aquaculture II: Effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J. Appl. Phycol. 1994, 6, 347–356. [Google Scholar] [CrossRef]
- Ahmad, I.; Hellebust, J.A. Protein-biosynthesis in salt-shocked cells of Stichococcus bacillaris (chlorophyceae). J. Phycol. 1993, 29, 294–300. [Google Scholar]
- Chakraborty, P.; Acharyya, T.; Babu, P.V.R.; Bandyopadhyay, D. Impact of salinity and pH on phytoplankton communities in a tropical freshwater system: An investigation with pigment analysis by HPLC. J. Environ. Monit. 2011, 13, 614–620. [Google Scholar] [CrossRef]
- Liska, A.J.; Shevchenko, A.; Pick, U.; Katz, A. Enhanced photosynthesis and redox energy production contribute to salinity tolerance in Dunaliella as revealed by homology-based proteomics. Plant Physiol. 2004, 136, 2806–2817. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Borowitzka, L.J.; Kessly, D. Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J. Appl. Phycol. 1990, 2, 111–119. [Google Scholar] [CrossRef]
- Ramos, A.; Polle, J.; Tran, D.; Cushman, J.C.; Jin, E.; Valera, J. The unicellular green alga Dunaliella salina Teod. as a model for abiotic stress tolerance: Genetic advances and future perspectives. Algae 2011, 26, 3–20. [Google Scholar] [CrossRef]
- Emerson, R.; Green, L. Effect of hydrogen-ion concentration on Chlorella photosynthesis. Plant Physiol. 1938, 13, 157–168. [Google Scholar] [CrossRef]
- Moss, B. The influence of environmental factors on the distribution of freshwater algae: An experimental study. II. The role of pH and the carbon dioxide- bicarbonate system. J. Ecol. 1973, 61, 157–177. [Google Scholar] [CrossRef]
- Azov, Y. Effect of pH on inorganic carbon uptake in algal cultures. Appl. Environ. Microbiol. 1982, 43, 1300–1306. [Google Scholar]
- Sunda, W.; Price, N.M.; Morel, F.M.M. Trace Metal Ion Buffers and Their Use in Culture Studies. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 35–64. [Google Scholar]
- Meseck, S.L.; Smith, B. How high pH’s can affect the chemistry in large volume cultures of Tetraselmis chui (PLY429). J. Shellfish Res. 2004, 23, 640. [Google Scholar]
- Smith, B.; Meseck, S. Some implications of controlling CO2 supply to cultures of Tetraselmis chui (PLY429). J. Shellfish Res. 2004, 23, 642. [Google Scholar]
- Goldman, J.C.; Azov, Y.; Riley, C.B.; Dennett, M.R. The effect of pH in intensive microalgal cultures.I. Biomass regulation. J. Exp. Mar. Biol. Ecol. 1982, 57, 1–13. [Google Scholar] [CrossRef]
- Goldman, J.C.; Riley, C.B.; Dennett, M.R. The effect of pH in intensive microalgal cultures. II. Species competition. J. Exp. Mar. Biol. Ecol. 1982, 57, 15–24. [Google Scholar] [CrossRef]
- Dimier, C.; Brunet, C.; Geider, R.; Raven, J. Growth and photoregulation dynamics of the picoeukaryote Pelagomonas calceolata in fluctuating light. Limnol. Oceanogr. 2009, 54, 823–836. [Google Scholar] [CrossRef]
- Schellenberger Costa, B.; Sachse, M.; Jungandreas, A.; Bartulos, C.R.; Gruber, A.; Jakob, T.; Kroth, P.G.; Wilhelm, C. Aureochrome 1a is involved in the photoacclimation of the diatom Phaeodactylum tricornutum. PLoS One 2013, 8, e74451–e74451. [Google Scholar] [CrossRef]
- Schellenberger Costa, B.; Jungandreas, A.; Jakob, T.; Weisheit, W.; Mittag, M.; Wilhelm, C. Blue light is essential for high light acclimation and photoprotection in the diatom Phaeodactylum tricornutum. J. Exp. Bot. 2013, 64, 483–493. [Google Scholar] [CrossRef]
- Torkamani, S.; Wani, S.N.; Tang, Y.J.; Sureshkumar, R. Plasmon-enhanced microalgal growth in miniphotobioreactors. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- Perrine, Z.; Negi, S.; Sayre, R.T. Optimization of photosynthetic light energy utilization by microalgae. Algal Res. 2012, 1, 134–142. [Google Scholar] [CrossRef]
- Murchie, E.H.; Pinto, M.; Horton, P. Agriculture and the new challenges for photosynthesis research. New Phytol. 2009, 181, 532–552. [Google Scholar] [CrossRef]
- Simionato, D.; Sforza, E.; Carpinelli, E.C.; Bertucco, A.; Giacometti, G.M.; Morosinotto, T. Acclimation of Nannochloropsis gaditana to different illumination regimes: Effects on lipids accumulation. Bioresour. Technol. 2011, 102, 6026–6032. [Google Scholar] [CrossRef]
- Van Leeuwe, M.A.; van Sikkelerus, B.; Gieskes, W.W.C.; Stefels, J. Taxon-specific differences in photoacclimation to fluctuating irradiance in an Antarctic diatom and a green flagellate. Mar. Ecol. Prog. Ser. 2005, 288, 9–19. [Google Scholar] [CrossRef]
- Litchman, E. Growth rates of phytoplankton under fluctuating light. Freshw. Biol. 2000, 44, 223–235. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Do light/dark cycles of medium frequency enhance phytoplankton productivity? J. Appl. Phycol. 1989, 1, 333–340. [Google Scholar] [CrossRef]
- Sforza, E.; Simionato, D.; Giacometti, G.M.; Bertucco, A.; Morosinotto, T. Adjusted light and dark cycles can optimize photosynthetic efficiency in algae growing in photobioreactors. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Paasche, E. Marine plankton algae grown with light-dark cycles. II. Ditylum brightwellii and Nitzschia turgidula. Physiol. Plant. 1968, 21, 66–77. [Google Scholar] [CrossRef]
- Brunet, C.; Davoult, D.; Casotti, R. Physiological reactions to a change in light regime in cultured Skeletonema costatum (Bacillariophyta): Implications for estimation of phytoplankton biomass. Hydrobiologia 1996, 333, 87–94. [Google Scholar] [CrossRef]
- Jacquet, S.; Partensky, F.; Lennon, J.F.; Vaulot, D. Diel patterns of growth and division in phytoplankton in culture. J. Phycol. 2001, 37, 357–369. [Google Scholar] [CrossRef]
- Clark, D.R.; Flynn, K.J.; Owens, N.J.P. The large capacity for dark nitrate-assimilation in diatoms may overcome nitrate limitation of growth. New Phytol. 2002, 155, 101–108. [Google Scholar] [CrossRef]
- Ashworth, J.; Coesel, S.; Lee, A.; Armbrust, E.V.; Orellana, M.V.; Baliga, N.S. Genome-wide diel growth state transitions in the diatom Thalassiosira pseudonana. Proc. Natl. Acad. Sci. USA 2013, 110, 7518–7523. [Google Scholar]
- Wagner, H.; Jakob, T.; Wilhelm, C. Balancing the energy flow from captured light to biomass under fluctuating light conditions. New Phytol. 2006, 169, 95–108. [Google Scholar] [CrossRef]
- Sanchez-Saavedra, M.P.; Voltolina, D. The growth rate, biomass production and composition of Chaetoceros sp. grown with different light sources. Aquac. Eng. 2006, 35, 161–165. [Google Scholar] [CrossRef]
- Marchetti, A.; Schruth, D.M.; Durkin, C.A.; Parker, M.S.; Kodner, R.B.; Berthiaume, C.T.; Morales, R.; Allen, A.E.; Armbrust, E.V. Comparative metatranscriptomics identifies molecular bases for the physiological responses of phytoplankton to varying iron availability. Proc. Natl. Acad. Sci. USA 2012, 109, E317–E325. [Google Scholar] [CrossRef]
- Huysman, M.J.J.; Fortunato, A.E.; Matthijs, M.; Costa, B.S.; Vanderhaeghen, R.; van den Daele, H.; Sachse, M.; Inze, D.; Bowler, C.; Kroth, P.G.; et al. AUREOCHROME1a-mediated induction of the diatom-specific cyclin dsCYC2 controls the onset of cell division in diatoms (Phaeodactylum tricornutum). Plant Cell 2013, 25, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Shihira-Ishikawa, I.; Nakamura, T.; Higashi, S.-i.; Watanabe, M. Distinct responses of chloroplasts to blue and green laser microbeam irradiations in the centric diatom Pleurosira laevis. Photochem. Photobiol. 2007, 83, 1101–1109. [Google Scholar] [CrossRef]
- Cao, S.; Wang, J.; Chen, D. Settlement and cell division of diatom Navicula can be influenced by light of various qualities and intensities. J. Basic Microbiol. 2013, 53, 884–894. [Google Scholar] [CrossRef]
- Brunet, C.; Chandrasekaran, R.; Barra, L.; Giovagnetti, V.; Corato, F.; Ruban, A.V. Spectral radiation dependent photoprotective mechanism in the diatom Pseudo-nitzschia multistriata. PLoS One 2014, 9, e87015. [Google Scholar]
- Brunet, C.; Conversano, F.; Margiotta, F.; Dimier, C.; Polimene, L.; Tramontano, F.; Saggiomo, V. Role of light and photophysiological properties on phytoplankton succession during the spring bloom in the north-western Mediterranean Sea. Adv. Oceanogr. Limnol. 2013, 4, 1–19. [Google Scholar] [CrossRef]
- Casotti, R.; Mazza, S.; Brunet, C.; Vantrepotte, V.; Ianora, A.; Miralto, A. Growth inhibition and toxicity of the diatom aldehyde 2-trans, 4-trans-decadienal on Thalassiosira weissflogii (bacillariophyceae). J. Phycol. 2005, 41, 7–20. [Google Scholar] [CrossRef]
- Wondraczek, L.; Batentschuk, M.; Schmidt, M.A.; Borchardt, R.; Scheiner, S.; Seemann, B.; Schweizer, P.; Brabec, C.J. Solar spectral conversion for improving the photosynthetic activity in algae reactors. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- BioProject. The National Center for Biotechnology Information (NCBI). Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?DB=genomeprj (accessed on 10 October 2013).
- Organelle Genome Resources. The National Center for Biotechnology Information (NCBI). Available online: http://www.ncbi.nlm.nih.gov/genomes/ORGANELLES/organelles.html (accessed on 10 October 2013).
- The Organelle Genome database (GOABASE). Available online: http://www.bch.umontreal.ca/gobase/gobase.html (accessed on 10 October 2013).
- Palenik, B.; Brahamsha, B.; Larimer, F.W.; Land, M.; Hauser, L.; Chain, P.; Lamerdin, J.; Regala, W.; Allen, E.E.; McCarren, J.; et al. The genome of a motile marine Synechococcus. Nature 2003, 424, 1037–1042. [Google Scholar] [CrossRef]
- Worden, A.Z.; Lee, J.-H.; Mock, T.; Rouzé, P.; Simmons, M.P.; Aerts, A.L.; Allen, A.E.; Cuvelier, M.L.; Derelle, E.; Everett, M.V. Green evolution and dynamic adaptations revealed by genomes of the marine picoeukaryotes Micromonas. Science 2009, 324, 268–272. [Google Scholar] [CrossRef]
- Vaulot, D.; Le Gall, F.; Marie, D.; Guillou, L.; Partensky, F. The Roscoff Culture Collection (RCC): A collection dedicated to marine picoplankton. Nova Hedwig. 2004, 79, 49–70. [Google Scholar] [CrossRef]
- Roscoff Culture Collection (RCC, Biological Station, Roscoff, France). Available online: http://www.sb-roscoff.fr/Phyto/RCC/index.php?option=com_frontpage&Itemid=1 (accessed on 10 October 2013).
- Marine Microbial Eukaryote Transcriptome Sequencing Project. Available online: http://marinemicroeukaryotes.org/project_organisms (accessed on 10 October 2013).
- Algae Industry Magazine. Available online: http://www.algaeindustrymagazine.com (accessed on 10 October 2013).
- Dunahay, T.G.; Jarvis, E.E.; Roessler, P.G. Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. J. Phycol. 1995, 31, 1004–1012. [Google Scholar]
- Falciatore, A.; Casotti, R.; Leblanc, C.; Abrescia, C.; Bowler, C. Transformation of nonselectable reporter genes in marine diatoms. Mar. Biotechnol. 1999, 1, 239–251. [Google Scholar] [CrossRef]
- Jin, E.; Polle, J.E.; Melis, A. Involvement of zeaxanthin and of the Cbr protein in the repair of photosystem II from photoinhibition in the green alga Dunaliella salina. BBA Bioenerg. 2001, 1506, 244–259. [Google Scholar] [CrossRef]
- Zaslavskaia, L.; Lippmeier, J.; Shih, C.; Ehrhardt, D.; Grossman, A.; Apt, K. Trophic conversion of an obligate photoautotrophic organism through metabolic engineering. Science 2001, 292, 2073–2075. [Google Scholar] [CrossRef]
- Doetsch, N.A.; Favreau, M.R.; Kuscuoglu, N.; Thompson, M.D.; Hallick, R.B. Chloroplast transformation in Euglena gracilis: Splicing of a group III twintron transcribed from a transgenic psbK operon. Curr. Genet. 2001, 39, 49–60. [Google Scholar] [CrossRef]
- Koksharova, O.; Wolk, C. Genetic tools for cyanobacteria. Appl. Microbiol. Biotechnol. 2002, 58, 123–137. [Google Scholar] [CrossRef]
- Nogales, J.; Gudmundsson, S.; Thiele, I. Toward systems metabolic engineering in cyanobacteria: Opportunities and bottlenecks. Bioengineered 2013, 4, 158–163. [Google Scholar] [CrossRef]
- Sayre, R.T.; Wagner, R.E.; Siripornadulsil, S.; Farias, C. Transgenic Algae for Delivering Antigens to an Animal. Patent US8,282,915 B2, 9 October 2012. [Google Scholar]
- Geng, D.G.; Wang, Y.Q.; Wang, P.; Li, W.B.; Sun, Y.R. Stable expression of hepatitis B surface antigen gene in Dunaliella salina (Chlorophyta). J. Appl. Phycol. 2003, 15, 451–456. [Google Scholar] [CrossRef]
- Sun, M.; Qian, K.X.; Su, N.; Chang, H.Y.; Liu, J.X.; Chen, G.F. Foot-and-mouth disease virus VP1 protein fused with cholera toxin B subunit expressed in Chlamydomonas reinhardtii chloroplast. Biotechnol. Lett. 2003, 25, 1087–1092. [Google Scholar] [CrossRef]
- Mayfield, S.P.; Franklin, S.E.; Lerner, R.A. Expression and assembly of a fully active antibody in algae. Proc. Natl. Acad. Sci. USA 2003, 100, 438–442. [Google Scholar] [CrossRef]
- Melis, A.; Happe, T. Hydrogen production. Green algae as a source of energy. Plant Physiol. 2001, 127, 740–748. [Google Scholar] [CrossRef]
- Rasala, B.A.; Mayfield, S.P. The microalga Chlamydomonas reinhardtii as a platform for the production of human protein therapeutics. Bioengineered 2011, 2, 50–54. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Sun, Y.; Zhang, L.; Li, W. Highly efficient expression of rabbit neutrophil peptide-1 gene in Chlorella ellipsoidea cells. Curr. Genet. 2001, 39, 365–370. [Google Scholar] [CrossRef]
- Hempel, F.; Lau, J.; Klingl, A.; Maier, U.G. Algae as protein factories: Expression of a human antibody and the respective antigen in the diatom Phaeodactylum tricornutum. PLoS One 2011, 6, e28424. [Google Scholar] [CrossRef]
- Jinkerson, R.E.; Radakovits, R.; Posewitz, M.C. Genomic insights from the oleaginous model alga Nannochloropsis gaditana. Bioengineered 2013, 4, 37–43. [Google Scholar] [CrossRef]
- Pan, K.; Qin, J.; Li, S.; Dai, W.; Zhu, B.; Jin, Y.; Yu, W.; Yang, G.; Li, D. Nuclear monoploidy and asexual propagation of Nannochloropsis oceanica (eustigmatophyceae) as revealed by its genome sequence. J. Phycol. 2011, 47, 1425–1432. [Google Scholar] [CrossRef]
- Depauw, F.A.; Rogato, A.; d’Alcalá, M.R.; Falciatore, A. Exploring the molecular basis of responses to light in marine diatoms. J. Exp. Bot. 2012, 63, 1575–1591. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barra, L.; Chandrasekaran, R.; Corato, F.; Brunet, C. The Challenge of Ecophysiological Biodiversity for Biotechnological Applications of Marine Microalgae. Mar. Drugs 2014, 12, 1641-1675. https://doi.org/10.3390/md12031641
Barra L, Chandrasekaran R, Corato F, Brunet C. The Challenge of Ecophysiological Biodiversity for Biotechnological Applications of Marine Microalgae. Marine Drugs. 2014; 12(3):1641-1675. https://doi.org/10.3390/md12031641
Chicago/Turabian StyleBarra, Lucia, Raghu Chandrasekaran, Federico Corato, and Christophe Brunet. 2014. "The Challenge of Ecophysiological Biodiversity for Biotechnological Applications of Marine Microalgae" Marine Drugs 12, no. 3: 1641-1675. https://doi.org/10.3390/md12031641