Maritime Halophyte Species from Southern Portugal as Sources of Bioactive Molecules
Abstract
:1. Introduction
2. Results and Discussion
2.1. RSA against DPPH (1,1-Diphenyl-2-picrylhydrazyl) and ABTS 2,2′-Azino-bis (3-Ethylbenzothiazoline-6-sulphonic Acid) Radicals, and TPC (Total Phenolic Content)
| Species/Compound | Extract | DPPH | ABTS | TPC |
|---|---|---|---|---|
| A. macrostachyum | Hexane | 5.0 ± 0.1 c | 9.6 ± 0.5 h | 39 ± 0.8 h |
| Diethyl ether | 0.3 ± 0.0 a | 2.7 ± 0.1 d,e | 33 ± 1.6 g | |
| Chloroform | 0.6 ± 0.1 a | 2.0 ± 0.0 c,d,e | 33 ± 0.4 g | |
| Methanol | 3.4 ± 0.1 b,c | 5.2 ± 0.2 g | 72 ± 0.5 k | |
| Water | >10 | >10 | 6.6 ± 0.2 a | |
| P. coronopus | Hexane | >10 | >10 | 5.8 ± 0.2 a |
| Diethyl ether | 8.9 ± 0.5 d | >10 | 16 ± 0.5 b,c | |
| Chloroform | >10 | >10 | 13 ± 0.2 b | |
| Methanol | 0.9 ± 0.1 a | 1.1 ± 0.1 a,b | 103 ± 1.8 m | |
| Water | 4.0 ± 1.1 c | 2.1 ± 0.0 d,e | 28 ± 0.2 f | |
| M. edule | Hexane | 5.3 ± 0.6 c | >10 | 4.5 ± 0.3 a |
| Diethyl ether | 1.8 ± 0.1 a,b | 2.9 ± 0.1 e | 22 ± 0.8 e | |
| Chloroform | >10 | 5.3 ± 0.0 f | 56 ± 0.7 j | |
| Methanol | 0.1 ± 0.0 a | 2.0 ± 0.0 c,d,e | 147 ± 0.6 n | |
| Water | 1.1 ± 0.3 a | 7.9 ± 0.2 g | 52 ± 1.5 j | |
| J. acutus | Hexane | 4.3 ± 0.3 c | 8.6 ± 0.3 g,h | 17 ± 0.3 c,d |
| Diethyl Ether | 0.2 ± 0.0 a | 0.4 ± 0.0 a | 93 ± 0.5 l | |
| Chloroform | >10 | 1.8 ± 0.3 b,c,d | 20 ± 0.3 g,h | |
| Methanol | 0.4 ± 0.0 a | 1.8 ± 0.1 b,c,d | 35 ± 0.2 i | |
| Water | >10 | 2.6 ± 0.1 d,e | 35 ± 1.0 g | |
| H. portulacoides | Hexane | >10 | >10 | 5.5 ± 0.1 a |
| Diethyl ether | >10 | 0.9 ± 0.0 a,b | 55 ± 0.7 j | |
| Chloroform | >10 | 4.0 ± 0.1 f | 13 ± 0.3 b | |
| Methanol | >10 | >10 | 15 ± 0.1 b,c | |
| Water | >10 | >10 | 21 ± 0.5 d,e | |
| BHT * | - | 0.1 ± 0.0 | 0.1 ± 0.0 | - |
2.2. Anti-Inflammatory Activity
2.3. Cytotoxic Activity

| Species/Compound | Extract | 3.9 µg/mL | 7.8 µg/mL | 15.6 µg/mL | 31.2 µg/mL | 62.5 µg/mL | 125 µg/mL | IC50 |
|---|---|---|---|---|---|---|---|---|
| A. macrostachyum | Water | 118 ± 3 *** | 117 ± 6 | 99 ± 4 | 103 ± 3 | 87 ± 4 | 77 ± 3 ** | n.d. |
| P. coronopus | Hexane | - | - | - | - | 56 ± 6 *** | 47 ± 3 *** | 98 ± 4.0 |
| Chloroform | 102 ± 5 | 97 ± 7 | 93 ± 8 | 90 ± 11 | - | - | n.d | |
| Methanol | - | - | - | 96 ± 3 | 97 ± 2 | 79 ± 3 *** | n.d | |
| Water | 112 ± 1 | 111 ± 2 | 110 ± 1 | 111 ± 2 | 103 ± 3 | 98 ± 3 | n.d | |
| H. portulacoides | Chloroform | - | - | - | 83 ± 3 | 73 ± 3 ** | 43 ± 1 *** | 109 ± 2.5 |
| J. acutus | Hexane | 102 ± 3 | - | - | - | - | - | n.d |
| Chloroform | 103 ± 7 | - | - | - | - | - | n.d | |
| M. edule | Methanol | 112 ± 4 | 114 ± 5 | 103 ± 3 | 115 ± 6 | 89 ± 5 | 111 ± 3 | n.d |
| Ether | 106 ± 3 | 104 ± 2 | 96 ± 2 | - | - | - | n.d | |
| Chloroform | 103 ± 2 | 89 ± 4 | 83 ± 6.6 | - | - | - | n.d | |
| Water | 144 ± 2 *** | 128 ± 6 ** | 169 ± 6 *** | 137 ± 7 *** | 137 ± 6 *** | 98 ± 6 | n.d | |
| L-NAME * | - | - | - | - | - | - | - | 29 ± 2.1 |
| Treatment | IC50 values | SI | |
|---|---|---|---|
| HepG2 | S17 | HepG2 | |
| Extract | 6.2 ± 0.3 * | 34 ± 2.2 | 5.5 |
| Etoposide | 1.9 ± 0.1 | 10 ± 0.01 | 5.4 |


| Cell lines | Etoposide | Juncunol | ||||
|---|---|---|---|---|---|---|
| IC50 | SI: S17 | SI: mTEC | IC50 | SI: S17 | SI: mTEC | |
| Tumor cell lines | ||||||
| HepG2 | 2.4 ± 0.2 a | 7.5 ± 0.4 B | 33 ± 2.3 G | 18 ± 1.2 b,c | 20 ± 1.4 F | 18 ± 1.2 E,F |
| MDA-MB-468 | 11 ± 0.7 c | 1.6 ± 0.1 A | 6.1 ± 0.7 B | 22 ± 2.0 b,c | 17 ± 1.7 E,F | 15 ± 1.6 D,E |
| HeLa | 7.1 ± 0.2 b | 2.5 ± 0.1 A | 10 ± 0.8 C | 27 ± 2.8 c | 13 ± 1.2 C,D | 12 ± 1.3 C,D |
| Non-tumor cell lines | ||||||
| S17 | 17 ± 0.2 d | - | - | 367 ± 3.2 g | - | - |
| mTEC | 75 ± 3.7 e | - | - | 344 ± 6.0 f | - | - |
3. Experimental Section
3.1. Reagents
3.2. Plant Material and Preparation of the Extracts
3.3. RSA by the DPPH• Assay
3.4. RSA by the ABTS•+ Assay
3.5. Total Phenolic Content (TPC)
3.6. Cell Culture
3.7. Quantification of NO
3.8. Cell Viability Assay
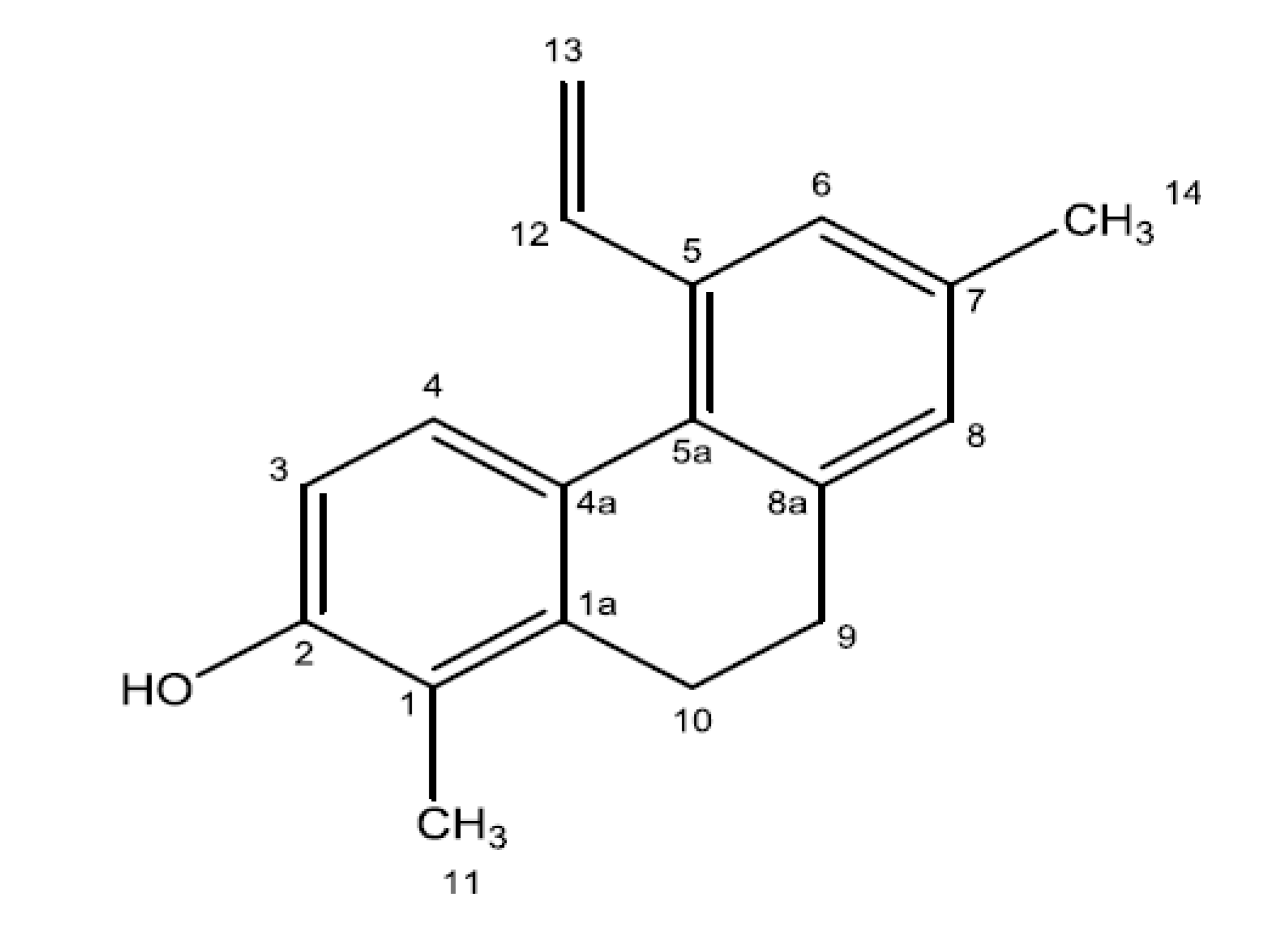
3.9. Bioguided Fractionation and Isolation of the Bioactive Compound
3.10. Spectral and Chromatographic Analysis
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Figueroa, M.E.; Davy, A.J. Salt stimulation of growth and photosynthesis in an extreme halophyte Arthrocnemum macrostachyum. Plant Biol. 2010, 12, 79–87. [Google Scholar]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Menezes-Benavente, L.; Teixeira, F.K.; Kamei, C.L.A.; Margis-Pinheiro, M. Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of a Brazilian indica rice (Oryza. sativa L.). Plant Sci. 2004, 166, 323–331. [Google Scholar] [CrossRef]
- Ben Amor, N.; Jiménez, A.; Megdiche, W.; Lundqvist, M.; Sevilla, F.; Abdelly, C. Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol. Plant. 2006, 126, 446–457. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Whid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Van der Watt, E.; Pretorius, J.C. Purification and identification of active antibacterial components in Carpobrotus edulis L. J. Ethnopharmacol. 2001, 76, 87–91. [Google Scholar] [CrossRef]
- Thring, T.S.A.; Weitz, F.M. Medicinal plant use in the Bredasdorp/Elim region of the Southern Overberg in the Western Cape Province of South Africa. J. Ethnopharmacol. 2006, 103, 261–275. [Google Scholar] [CrossRef]
- Bouftira, I.; Abdelly, C.; Sfar, S. Identification of a naturally occurring 2, 6-bis (1,1-dimethylethyl)-4-methylphenol from purple leaves of the halophyte plant Mesembryanthemum crystallinum. Afr. J. Biotechnol. 2007, 6, 1136–1139. [Google Scholar]
- Falleh, H.; Ksouri, R.; Medini, F.; Guyot, S.; Abdelly, C.; Magné, C. Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Ind. CropsProd. 2011, 34, 1066–1071. [Google Scholar]
- Falleh, H.; Jalleli, I.; Ksouri, R.; Boulaaba, M.; Guyot, S.; Magné, C.; Abdelly, C. Effect of salt treatment on phenolic compounds and antioxidant activity of two Mesembryanthemum edule provenances. Plant Physiol. Biochem. 2012, 52, 1–8. [Google Scholar] [CrossRef]
- Custódio, L.; Ferreira, A.C.; Pereira, H.; Silvestre, L.; Vizetto-Duarte, C.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. The marine halophytes Carpobrotus edulis L. and Arthrocnemum macrostachyum L. are potential sources of nutritionally important PUFAs and metabolites with antioxidant, metal chelating and anticholinesterase inhibitory activities. Bot. Mar. 2012, 55, 281–288. [Google Scholar]
- Awaad, A.S. Phenolic glycosides of Juncus acutus and its anti-eczematic activity. Chem. Nat. Compd. 2006, 42, 152–155. [Google Scholar] [CrossRef]
- Behery, F.A.; Naeema, Z.E.; Maatooq, G.T.; Amer, M.M.; Ahmed, A.F. A novel antioxidant phenanthrenoid dimer from Juncus acutus L. Nat. Prod. Res. 2013, 27, 155–163. [Google Scholar] [CrossRef]
- El-Wahab, R.H.A.; Zaghloul, M.S.; Kamel, W.M.; Moustafa, A.R.A. Diversity and distribution of medicinal plants in North Sinai, Egypt. Afr. J. Environ. Sci. Technol. 2008, 2, 157–171. [Google Scholar]
- Benzarti, M.; Rejeb, K.B.; Debez, A.; Messedi, D.; Abdelly, C. Photosynthetic activity and leaf antioxidative responses of Atriplex portulacoides subjected to extreme salinity. Acta Physiol. Plant. 2012, 34, 1679–1688. [Google Scholar] [CrossRef]
- Grossi, V.; Raphel, D. Long-chain (C-19-C-29) 1-chloro-n-alkanes in leaf waxes of halophytes of the Chenopodiaceae. Phytochemistry 2003, 63, 693–698. [Google Scholar]
- Oliveira, V.; Santos, A.L.; Aguiar, C.; Santos, L.; Salvador, A.C.; Gomes, N.C.M.; Silva, H.; Rocha, S.M.; Almeida, A.; Cunha, A. Prokaryotes in salt marsh sediments of Ria de Aveiro: Effects of halophyte vegetation on abundance and diversity. Estuar. Coast. Shelf Sci. 2012, 110, 61–68. [Google Scholar] [CrossRef]
- Vilela, C.; Santos, S.A.O.; Coelho, D.; Silva, A.M.S.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Screening of lipophilic and phenolic extractives from different morphological parts of Halimione portulacoides. Ind. Crop. Prod. 2014, 32, 373–379. [Google Scholar]
- Trabelsi, N.; Falleh, H.; Inès, J.; Daly, A.B.; Hajlaoui, H.; Smaoui, A.; Abdelly, C.; Ksouri, R. Variation of phenolic composition and biological activities in Limoniastrum monopetalum L. organs. Acta Physiol. Plant. 2012, 34, 87–96. [Google Scholar] [CrossRef]
- Trabelsi, N.; Waffo-Téguo, P.; Snoussi, M.; Ksouri, R.; Mérillon, J.M.; Smaoui, A.; Abdelly, C. Variability of phenolic composition and biological activities of two Tunisian halophyte species from contrasted regions. Acta Physiol. Plant. 2013, 35, 749–761. [Google Scholar] [CrossRef]
- Conforti, F.; Sosa, S.; Marrelli, M.; Menichini, F.; Statti, G.A.; Uzunov, D.; Tubaro, A.; Menichini, F.; Loggia, R.D. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J. Ethnopharmacol. 2008, 116, 144–151. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Kubes, P.; McCafferty, D.M. Nitric oxide and intestinal inflammation. Am. J. Med. 2000, 109, 150–158. [Google Scholar] [CrossRef]
- Beara, I.N.; Lesjak, M.M.; Orčić, D.Z.; Simin, N.Ð.; Četojević-Simin, D.D.; Božin, B.N.; Mimica-Dukić, N.M. Comparative analysis of phenolic profile, antioxidant, anti-inflammatory and cytotoxic activity of two closely-related Plantain species: Plantago altissima L. and Plantago lanceolata L. LWT Food Sci. Technol. 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Ordway, D.; Hohmann, J.; Viveiros, M.; Viveiros, A.; Leandro, C.; Arroz, M.J.; Gracio, M.A.; Amaral, L. Carpobrotus edulis methanol extract inhibits the MDR efflux pumps, enhances killing of phagocytosed S. aureus and promotes immune modulation. Phytother. Res. 2003, 17, 512–519. [Google Scholar] [CrossRef]
- Martins, M.; Ordway, D.; Kristiansen, M.; Viveiros, M.; Leandro, C.; Molnar, J.; Amaral, L. Inhibition of the Carpobrotus. edulis methanol extract on the growth of phagocytosed multidrug-resistant Mycobacterium tuberculosis and methicillin-resistant Staphylococcus aureus. Fitoterapia 2005, 76, 96–99. [Google Scholar] [CrossRef]
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants used against cancer—An extension of the work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef]
- Gálvez, M.; Martín-Cordero, C.; López-Lázaro, M.; Cortés, F.; Ayuso, M.J. Cytotoxic effect of Plantago. spp. on cancer cell lines. J. Ethnopharmacol. 2003, 88, 125–130. [Google Scholar] [CrossRef]
- Martins, A.; Vasas, A.; Schelz, Z.; Viveiros, M.; Molnár, J.; Hohmann, J.; Amaral, L. Constituents of Carpobrotus edulis inhibit P-glycoprotein of MDR1-transfected mouse lymphoma cells. Anticancer Res. 2010, 30, 829–835. [Google Scholar]
- El-Shamy, A.I.; Abdel-Razek, A.F.; Nassar, M.I. Phytochemical review of Juncus L. genus (Fam. Juncaceae). Arab. J. Chem. 2012. [Google Scholar] [CrossRef]
- Martinez-Lirola, M.J.; Gonzalez-Tejero, M.R.; Molero-Mesa, J. Ethnobotanical resources in the province of Almeria, Spain, Campos De Nijar. Econ. Bot. 1996, 50, 40–56. [Google Scholar] [CrossRef]
- Behery, F.A.; Naeem, Z.E.; Maatooq, G.T.; Amer, M.M.; Wen, Z.H.; Sheu, J.H.; Ahmed, A.F. Phenanthrenoids from Juncus acutus L., new natural lipopolysaccharide-inducible nitric oxide synthase inhibitors. Chem. Pharm. Bull. 2007, 55, 1264–1266. [Google Scholar] [CrossRef]
- Miles, H.D.; Bhattacharyya, J.; Mody, J.L.; Atwood, S.B.; Hedin, P.A. The structure of juncusol. A novel cytotoxic dihydrophenanthrene from estuarine marsh plant Juncus roemerianus. J. Am. Chem. Soc. 1977, 99, 618–620. [Google Scholar] [CrossRef]
- Dos Santos Júnior, H.M.; Oliveira, D.; de Carvalho, D.A.; Pinto, J.; Campos, V.; Mourão, A.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Evaluation of native and exotic Brazilian plants for anticancer activity. J. Nat. Med. 2010, 64, 231–238. [Google Scholar] [CrossRef]
- Della Greca, M.; Fiorentino, A.; Mangoni, L.; Molinaro, A.; Monaco, P.; Previtera, L. 9,10-dihydrophenanthrene metabolites from Juncus effusus L. Tetrahedron Lett. 1992, 33, 5257–5260. [Google Scholar]
- Della Greca, M.; Fiorentino, A.; Mangoni, A.; Molinaro, A.; Monaco, P.; Previtera, L. A bioactive dihydrodibenzoxepin from Juncus effusus. Phytochemistry 1993, 34, 1182–1184. [Google Scholar] [CrossRef]
- DellaGreca, M.; Isidori, M.; Lavorgna, M.; Monaco, P.; Previtera, L.; Zarrelli, A. Bioactivity of phenanthrenes from Juncus acutus on Selenastrum capricornutum. J. Chem. Ecol. 2004, 30, 867–879. [Google Scholar] [CrossRef]
- Sarkar, H.; Zerezghi, M.; Bhattacharyya, J. Dehydrojuncusol, a constituent of the roots of Juncus roemerianus. Phytochemistry 1988, 27, 3006–3008. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Nishishiro, M.; Arikawa, S.; Wakabayashi, H.; Hashimoto, K.; Saton, K.; Yokoyama, K.; Unten, S.; Kakuta, H.; Kurihara, T.; Motohashi, N.; et al. Inhibition of LPS-stimulated NO production in mouse macrophage-like cells by azulenequinones. Anticancer Res. 2005, 25, 4157–4163. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Oh, S.H.; Ahn, J.; Kang, D.H.; Lee, H.Y. The effect of ultrasonificated extracts of Spirulina maxima on the anticancer activity. Mar. Biotechnol. 2011, 13, 205–214. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Li, H.; Yu, M.; Gao, J.; Wang, L.; Zhai, Y. Cytotoxicity and apoptosis induced by a new podophyllotoxin glucoside in human hepatoma (HepG2) cells. Can. J. Physiol. Pharmacol. 2010, 88, 472–479. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime Halophyte Species from Southern Portugal as Sources of Bioactive Molecules. Mar. Drugs 2014, 12, 2228-2244. https://doi.org/10.3390/md12042228
Rodrigues MJ, Gangadhar KN, Vizetto-Duarte C, Wubshet SG, Nyberg NT, Barreira L, Varela J, Custódio L. Maritime Halophyte Species from Southern Portugal as Sources of Bioactive Molecules. Marine Drugs. 2014; 12(4):2228-2244. https://doi.org/10.3390/md12042228
Chicago/Turabian StyleRodrigues, Maria João, Katkam N. Gangadhar, Catarina Vizetto-Duarte, Sileshi G. Wubshet, Nils T. Nyberg, Luísa Barreira, João Varela, and Luísa Custódio. 2014. "Maritime Halophyte Species from Southern Portugal as Sources of Bioactive Molecules" Marine Drugs 12, no. 4: 2228-2244. https://doi.org/10.3390/md12042228





