Omega-3 Polyunsaturated Fatty Acids Protect Neural Progenitor Cells against Oxidative Injury
Abstract
:1. Introduction
2. Results
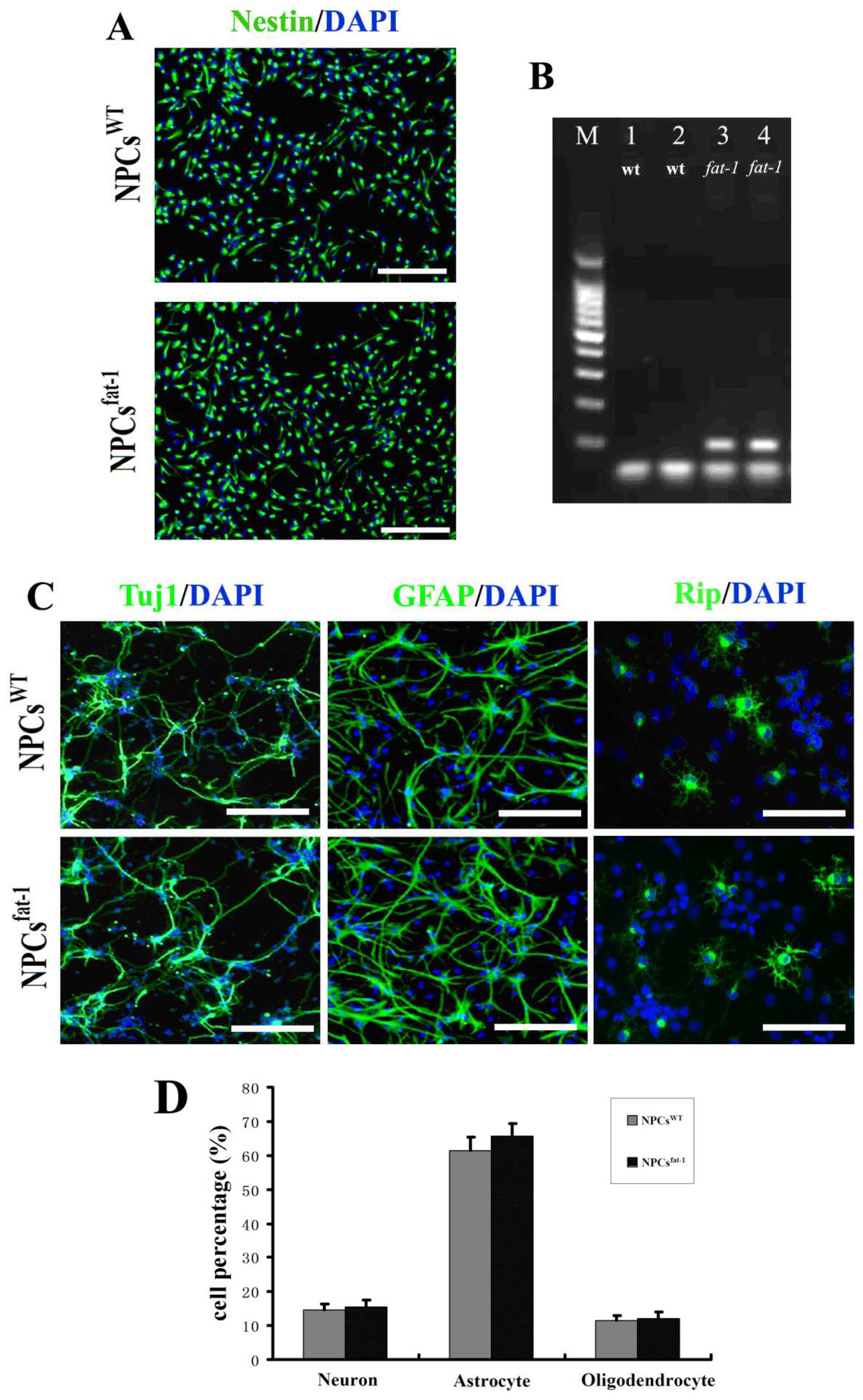
2.1. In Vitro Characterization of NPCsWT and NPCsfat-1
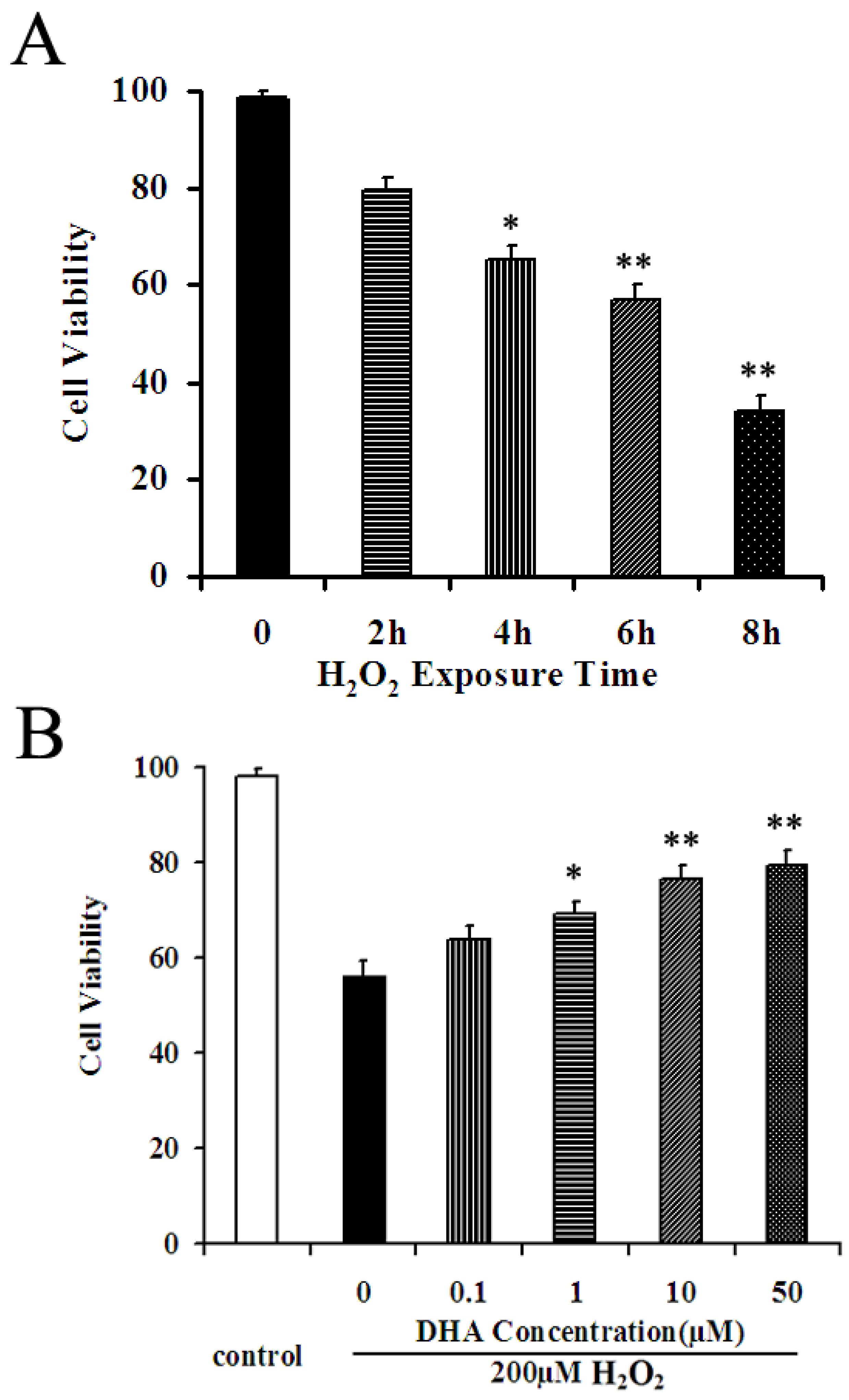
2.2. DHA Protected NPCs against H2O2-Mediated Apoptosis
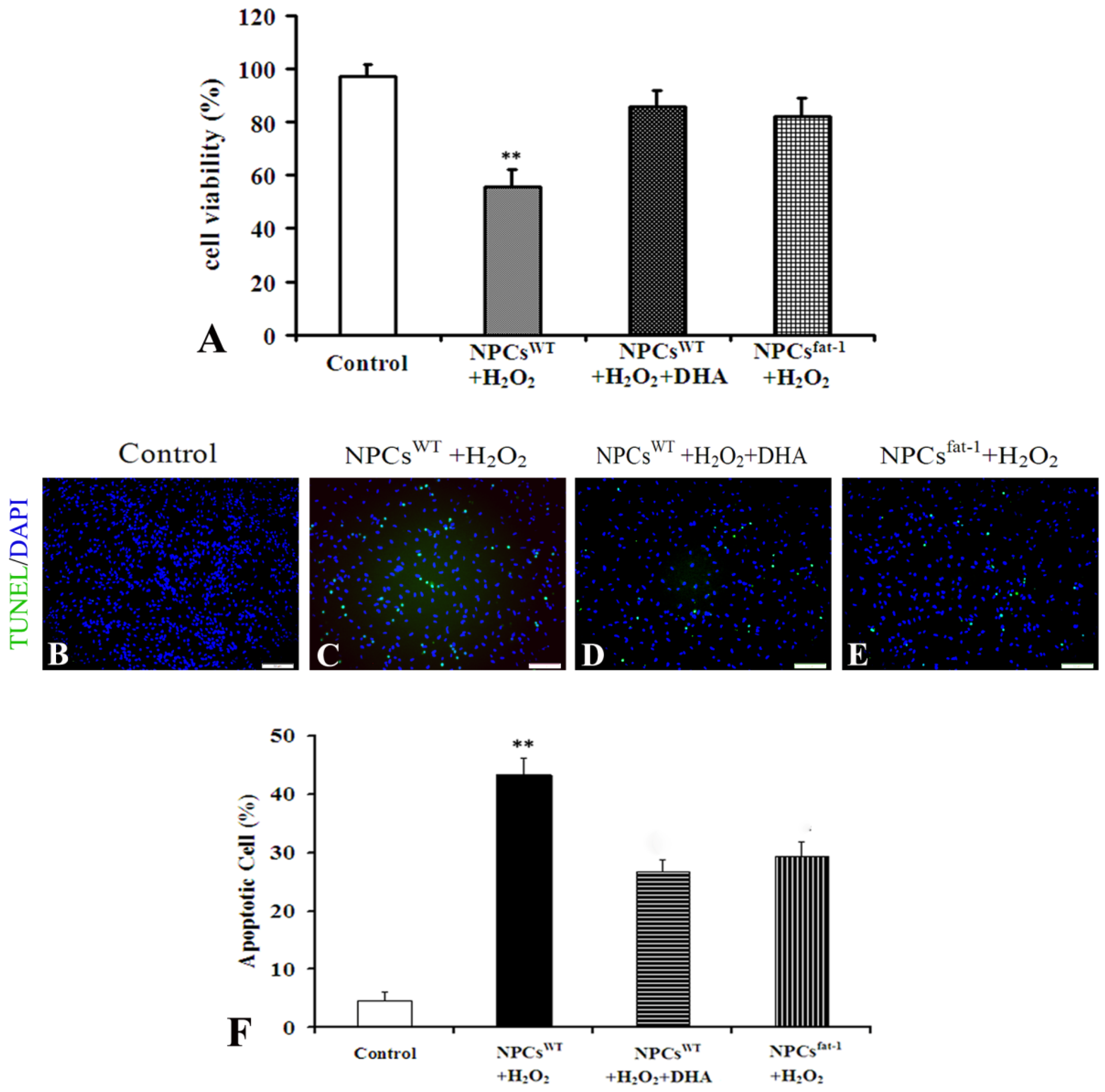
2.3. NPCsfat-1 Prevented H2O2-Mediated Apoptosis
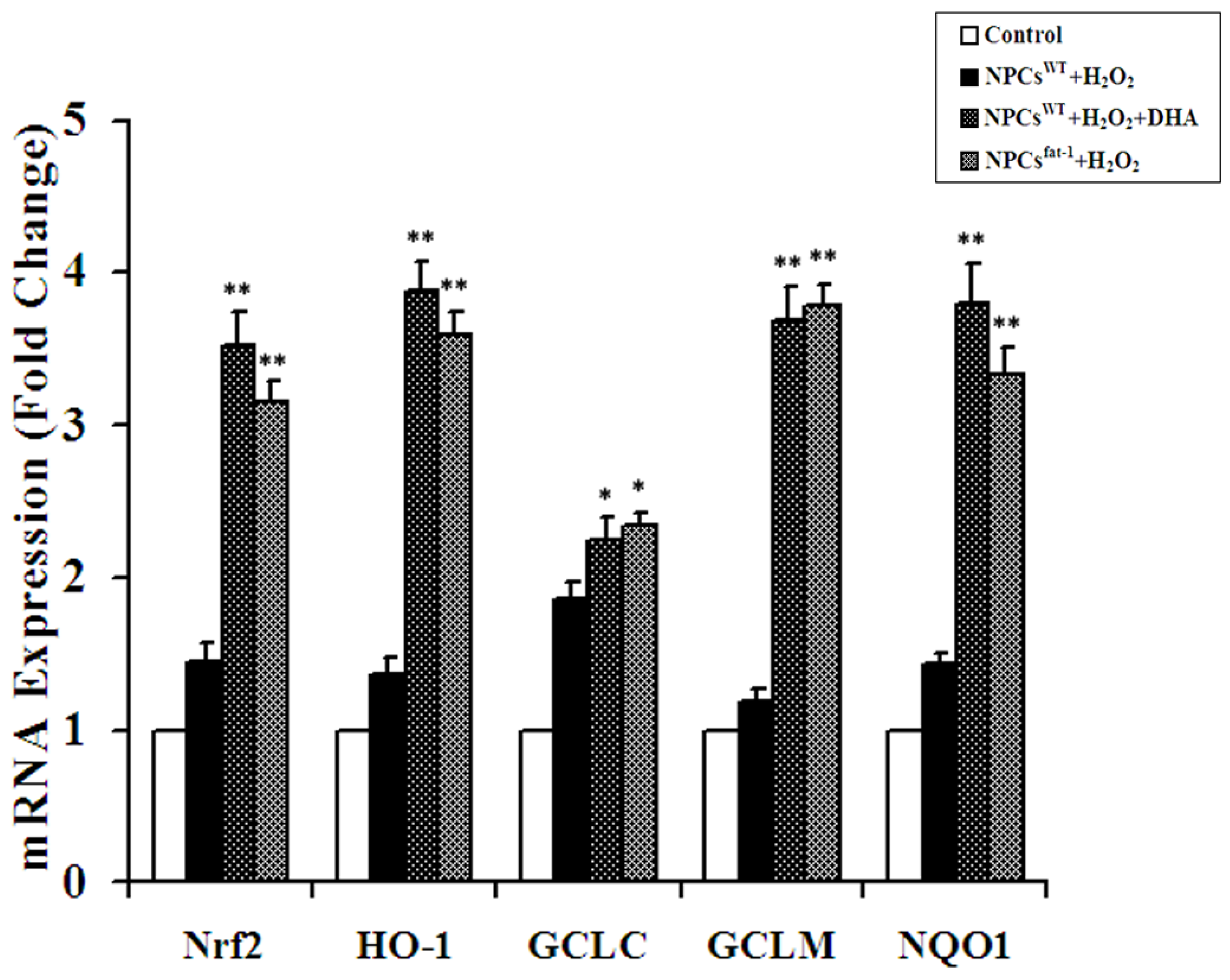
2.4. Expression Analyses of Nrf2-ARE Pathway Genes
2.5. Expression Profiles of Nuclear and Cytosolic Nrf2 by Western Blot Analysis
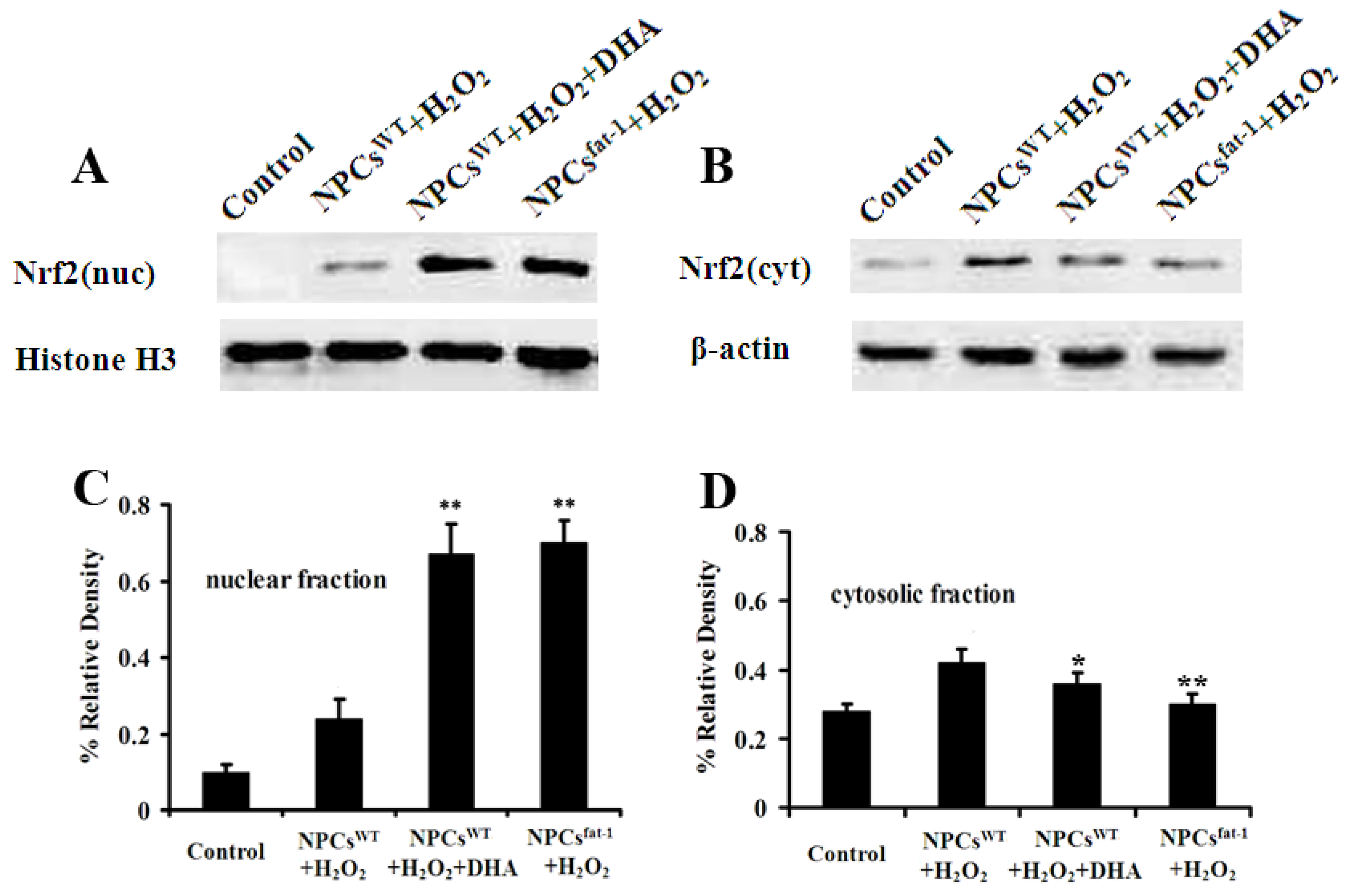
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Genomic DNA Extractions and PCR Amplification
4.3. Cell Isolation and Culture
4.4. Exposure to H2O2 and Pretreatment with DHA
4.5. Analysis of Cell Viability and TUNEL
4.6. Real-Time RT-PCR
| Gene | Primer (5′-3′) |
|---|---|
| Nrf2 | F: TTCTTTCAGCAGCATCCTCTCCAC |
| R: ACAGCCTTCAATAGTCCCGTCCAG | |
| NQO1 | F: GCGAGAAGAGCCCTGATTGTACTG |
| R: TCTCAAACCAGCCTTTCAGAATGG | |
| HO-1 | F: CAAGCCGAGAATGCTGAGTTCATG |
| R: GCAAGGGATGATTTCCTGCCAG | |
| GCLM | F: GCCACCAGATTTGACTGCCTTTG |
| R: TGCTCTTCACGATGACCGAGTACC | |
| GCLC | F: ACATCTACCACGCAGTCAAGGACC |
| R: CTCAAGAACATCGCCTCCATTCAG | |
| β-actin | F: TCGTGCGTGACATTAAGGAGAAG |
| R: GTTGAAGGTAGTTTCGTGGATGC |
4.7. Immunofluorescence
4.8. Western Blotting Analysis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Xu, J.; Kao, S.Y.; Lee, F.J.; Song, W.; Jin, L.W.; Yankner, B.A. Dopamine-dependent neurotoxicity of alpha-synuclein: A mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 2002, 8, 600–606. [Google Scholar] [CrossRef]
- Zahler, S.; Kupatt, C.; Becker, B.F. Endothelial preconditioning by transient oxidative stress reduces inflammatory responses of cultured endothelial cells to TNF-alpha. FASEB J. 2000, 14, 555–564. [Google Scholar]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Chang, Y.L.; Chen, S.J.; Kao, C.L.; Hung, S.C.; Ding, D.C.; Yu, C.C.; Chen, Y.J.; Ku, H.H.; Lin, C.P.; Lee, K.H.; et al. Docosahexaenoic acid promotes dopaminergic differentiation in induced pluripotent stem cells and inhibits teratoma formation in rats with Parkinson-like pathology. Cell Transplant. 2012, 21, 313–332. [Google Scholar] [CrossRef]
- Russell, F.D.; Bürgin-Maunder, C.S. Distinguishing health benefits of eicosapentaenoic and docosahexaenoic acids. Mar. Drugs. 2012, 10, 2535–2559. [Google Scholar] [CrossRef]
- Tixier-Vidal, A.; Picart, R.; Loudes, C.; Bauman, A.F. Effects of polyunsaturated fatty acids and hormones on synaptogenesis in serum-free medium cultures of mouse fetal hypothalamic cells. Neuroscience 1986, 17, 115–132. [Google Scholar] [CrossRef]
- Greiner, R.S.; Moriguchi, T.; Hutton, A.; Slotnick, B.M.; Salem, N., Jr. Rats with low levels of brain docosahexaenoic acid show impaired performance in olfactory-based and spatial learning tasks. Lipids 1999, 34, S239–S243. [Google Scholar] [CrossRef]
- Gamoh, S.; Hashimoto, M.; Sugioka, K.; Hossain, M.S.; Hata, N.; Misawa, Y.; Masumura, S. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience 1999, 93, 237–241. [Google Scholar] [CrossRef]
- Gamoh, S.; Hashimoto, M.; Hossain, S.; Masumura, S. Chronic administration of docosahexaenoic cid improves the performance of radial arm maze task in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 266–270. [Google Scholar] [CrossRef]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N., Jr.; Frautschy, S.A.; Cole, G.M. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, Y.G.; Baik, E.J.; Jung, S.J.; Won, R.; Nahm, T.S.; Lee, B.H. Dehydroascorbic acid prevents oxidative cell death through a glutathione pathway in primary astrocytes. J. Neurosci. Res. 2005, 79, 670–679. [Google Scholar] [CrossRef]
- Kang, Z.B.; Ge, Y.; Chen, Z.; Cluette-Brown, J.; Laposata, M.; Leaf, A.; Kang, J.X. Adenoviral gene transfer of Caenorhabditis elegans n-3 fatty acid desaturase optimizes fatty acid composition in mammalian cells. Proc. Natl. Acad. Sci. USA 2001, 98, 4050–4054. [Google Scholar] [CrossRef]
- Kang, J.X.; Wang, J.; Wu, L.; Kang, Z.B. Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004, 427. [Google Scholar] [CrossRef]
- Xia, S.; Lu, Y.; Wang, J.; He, C.; Hong, S.; Serhan, C.N.; Kang, J.X. Melanoma growth is reduced in fat-1 transgenic mice: Impact of omega-6/omega-3 essential fatty acids. Proc. Natl. Acad. Sci. USA 2006, 103, 12499–12504. [Google Scholar] [CrossRef]
- Agar, J.; Durham, H. Relevance of oxidative injury in the pathogenesis of motor neuron diseases. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2003, 4, 232–242. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar]
- Lin, H.J.; Wang, X.; Shaffer, K.M.; Sasaki, C.Y.; Ma, W. Characterization of H2O2-induced acute apoptosis in cultured neural stem/progenitor cells. FEBS Lett. 2004, 570, 102–106. [Google Scholar] [CrossRef]
- Brand, A.; Schonfeld, E.; Isharel, I.; Yavin, E. Docosahexaenoic acid-dependent iron accumulation in oligodendroglia cells protects from hydrogen peroxide-induced damage. J. Neurochem. 2008, 105, 1325–1335. [Google Scholar] [CrossRef]
- Shimazawa, M.; Nakajima, Y.; Mashima, Y.; Hara, H. Docosahexaenoic acid (DHA) has neuroprotective effects against oxidative stress in retinal ganglion cells. Brain Res. 2009, 1251, 269–275. [Google Scholar] [CrossRef]
- Bechoua, S.; Dubois, M.; Dominguez, Z.; Goncalves, A.; Némoz, G.; Lagarde, M.; Prigent, A.F. Protective effect of docosahexaenoic acid against hydrogen peroxide-induced oxidative stress in human lymphocytes. Biochem. Pharmacol. 1999, 57, 1021–1030. [Google Scholar] [CrossRef]
- He, C.; Qu, X.; Cui, L.; Wang, J.; Kang, J.X. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. USA 2009, 106, 11370–11375. [Google Scholar] [CrossRef]
- Su, H.X.; Zhang, W.M.; Guo, J.S.; Guo, A.C.; Yuan, Q.J.; Wu, W.T. Neural Progenitor Cells Enhance the Survival and Axonal Regeneration of Injured Motoneurons after Transplantation into the Avulsed Ventral Horn of Adult Rats. J. Neurotrauma 2009, 26, 67–80. [Google Scholar] [CrossRef]
- Zhao, Z.; Wen, H.; Fefelova, N.; Allen, C.; Guillaume, N.; Xiao, D.; Huang, C.; Zang, W.; Gwathmey, J.K.; Xie, L.H. Docosahexaenoic Acid reduces the incidence of early afterdepolarizations caused by oxidative stress in rabbit ventricular myocytes. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef]
- Lee, J.M.; Johnson, J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar] [CrossRef]
- Ryu, J.; Zhang, R.; Hong, B.H.; Yang, E.J.; Kang, K.A.; Choi, M.; Kim, K.C.; Noh, S.J.; Kim, H.S.; Lee, N.H.; et al. Phloroglucinol attenuates motor functional deficits in an animal model of Parkinson’s disease by enhancing Nrf2 activity. PLoS One 2013, 8, e71178. [Google Scholar] [CrossRef]
- Kanninen, K.; Heikkinen, R.; Malm, T.; Rolova, T.; Kuhmonen, S.; Leinonen, H.; Ylä-Herttuala, S.; Tanila, H.; Levonen, A.L.; Koistinaho, M.; et al. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 16505–16510. [Google Scholar] [CrossRef]
- Nanou, A.; Higginbottom, A.; Valori, C.F.; Wyles, M.; Ning, K.; Shaw, P.; Azzouz, M. Viral delivery of antioxidant genes as a therapeutic strategy in experimental models of amyotrophic lateral sclerosis. Mol. Ther. 2013, 21, 1486–1496. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lii, C.K.; Wei, Y.L.; Li, C.C.; Lu, C.Y.; Liu, K.L.; Chen, H.W. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-κB pathways. J. Nutr. Biochem. 2013, 24, 204–212. [Google Scholar] [CrossRef]
- Stulnig, G.; Frisch, M.T.; Crnkovic, S.; Stiegler, P.; Sereinigg, M.; Stacher, E.; Olschewski, H.; Olschewski, A.; Frank, S. Docosahexaenoic acid (DHA)-induced heme oxygenase-1 attenuates cytotoxic effects of DHA in vascular smooth muscle cells. Atherosclerosis 2013, 230, 406–413. [Google Scholar] [CrossRef]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Yamashima, T. A putative link of PUFA, GPR40 and adult-born hippocampal neurons for memory. Prog. Neurobiol. 2008, 84, 105–115. [Google Scholar] [CrossRef]
- Lafourcade, M.; Larrieu, T.; Mato, S.; Duffaud, A.; Sepers, M.; Matias, I.; De Smedt-Peyrusse, V.; Labrousse, V.F.; Bretillon, L.; Matute, C.; et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011, 14, 345–350. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Q.; Wu, D.; Ni, N.; Ren, H.; Luo, C.; He, C.; Kang, J.-X.; Wan, J.-B.; Su, H. Omega-3 Polyunsaturated Fatty Acids Protect Neural Progenitor Cells against Oxidative Injury. Mar. Drugs 2014, 12, 2341-2356. https://doi.org/10.3390/md12052341
Liu Q, Wu D, Ni N, Ren H, Luo C, He C, Kang J-X, Wan J-B, Su H. Omega-3 Polyunsaturated Fatty Acids Protect Neural Progenitor Cells against Oxidative Injury. Marine Drugs. 2014; 12(5):2341-2356. https://doi.org/10.3390/md12052341
Chicago/Turabian StyleLiu, Qiang, Di Wu, Na Ni, Huixia Ren, Chuanming Luo, Chengwei He, Jing-Xuan Kang, Jian-Bo Wan, and Huanxing Su. 2014. "Omega-3 Polyunsaturated Fatty Acids Protect Neural Progenitor Cells against Oxidative Injury" Marine Drugs 12, no. 5: 2341-2356. https://doi.org/10.3390/md12052341




