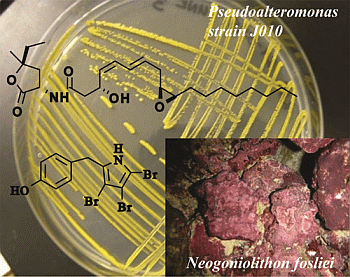
A Coralline Algal-Associated Bacterium, Pseudoalteromonas Strain J010, Yields Five New Korormicins and a Bromopyrrole
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation of Bacterial Metabolites
| Position | δC, mult. | δH (J Hz) | gCOSY | gHMBC a |
|---|---|---|---|---|
| NH | - | - | - | - |
| 2 | 131.1, qC | - | - | - |
| 3 | 97.8, qC | - | - | - |
| 4 | 100.0, qC | - | - | - |
| 5 | 100.0, qC | - | - | - |
| 6 | 32.5, CH2 | 3.87, s | - | 2, 3, 7, 8, 12 |
| 7 | 128.7, qC | - | - | - |
| 8 | 129.9, CH | 7.05, d (8.3) | 9 | 2 b, 6, 7, 9, 10, 11, 12 |
| 9 | 115.8, CH | 6.81, d (8.3) | 8 | 8, 10, 11, 12 |
| 10 | 155.1, qC | - | - | - |
| OH | - | - | - | - |
| 11 | 115.8, CH | 6.81, d (8.3) | 12 | 8, 10, 11, 12 |
| 12 | 129.9, CH | 7.05, d (8.3) | 11 | 6, 8, 9, 10, 11 |

| No. | Korormicin | |||||
|---|---|---|---|---|---|---|
| G (2) | H (3) | I (4) | I (4) | J (5) | K (6) | |
| δC, mult. a | δC, mult. b | δC, mult. b | δC, mult. a | δC, mult. a | δC, mult. a | |
| 1 | - | - | - | - | - | - |
| 2 | 174.7, qC | 168.5, qC | 168.5, qC | 168.5, qC | nd c | 174.3, qC |
| 3 | 50.0, CH | 125.0, qC | 125.0, qC | 125.0, qC | 50.0, CH | 50.4, CH |
| 4 | 39.9, CH2 | 133.7, CH | 134.0, CH | 134.0, CH | 40.0, CH2 | 40.4, CH2 |
| 5 | 85.8, qC | 87.2, qC | 87.2, qC | 87.2, qC | nd c | 85.6, qC |
| 6 | 32.4, CH2 | 31.2, CH2 | 31.1, CH2 | 34.6, CH2 | 32.9, CH2 | 32.8, CH2 |
| 7 | 8.2, CH3 | 8.0, CH3 | 8.0, CH3 | 8.1, CH3 | 8.3, CH3 | 8.4, CH3 |
| 8 | 25.9, CH3 | 23.9, CH3 | 24.1, CH3 | 24.2, CH3 | 25.9, CH3 | 26.2, CH3 |
| NH | - | - | - | - | - | - |
| 1′ | 172.0, qC | 170.1, qC | 170.2, qC | 170.2, qC | nd c | 171.7, qC |
| 2′ | 42.3, CH2 | 43.9, CH2 | 44.0, CH2 | 43.1, CH2 | 42.7, CH2 | 42.7, CH2 |
| 3′ | 64.7, CH | 63.5, CH | 63.8, CH | 64.6, CH | 64.8, CH | 65.0, CH |
| OH | - | - | - | - | - | - |
| 4′ | 129.3 CH | 132.8 CH | 132.2 CH | 129.4, CH | 127.6, CH | 129.0 CH |
| 5′ | 130.0, CH | 128.2, CH | 128.5, CH | 130.7, CH | 130.6, CH | 131.0, CH |
| 6′ | 126.4, CH | 127.8, CH | 127.4, CH | 127.4, CH | 124.0, CH | 124.7, CH |
| 7′ | 132.0, CH | 131.9, CH | 132.5, CH | 132.7, CH | 136.6, CH | 136.4, CH |
| 8′ | 31.1, CH2 | 37.2, CH2 | 37.1, CH2 | 38.2, CH2 | 30.5, CH2 | 30.6, CH2 |
| 9′ | 55.3, CH | 67.0, CH | 72.1, CH | 73.1, CH | 128.3, CH | 126.1, CH |
| OH | - | - | - | - | - | - |
| 10′ | 56.6, CH | 71.4, CH | 67.7, CH | 68.0, CH | 129.2 CH | 131.4, CH |
| 11′ | 27.3, CH2 | 33.3, CH2 | 33.7, CH2 | 31.9, CH2 | 27.0, CH2 | 27.2, CH2 |
| 12′ | 29.1 d, CH2 | 25.4, CH2 | 26.3, CH2 | 26.0, CH2 | 25.4, CH2 | 29.1 d, CH2 |
| 13′ | 29.1 d, CH2 | 29.0 d, CH2 | 28.8 d, CH2 | 28.8 d, CH2 | 29.0 d, CH2 | 29.1 d, CH2 |
| 14′ | 29.1 d, CH2 | 29.0 d, CH2 | 28.6 d, CH2 | 28.58 d, CH2 | 28.6 d, CH2 | 29.1 d, CH2 |
| 15′ | 29.1 d, CH2 | 29.0, CH2 | 28.6 d, CH2 | 28.55 d, CH2 | 31.4, CH2 | 29.1 d, CH2 |
| 16′ | 31.6, CH2 | 31.3, CH2 | 31.2, CH2 | 31.7, CH2 | 22.0, CH2 | 31.6, CH2 |
| 17′ | 22.6, CH2 | 22.1, CH2 | 22.1, CH2 | 22.6, CH2 | 13.5, CH3 | 22.6, CH2 |
| 18′ | 13.9, CH3 | 13.9, CH3 | 13.9, CH3 | 13.9, CH3 | - | 14.1, CH3 |
| No. | Korormicin | |||||
|---|---|---|---|---|---|---|
| G (2) | H (3) | I (4) | I (4) | J (5) | K (6) | |
| δH (J Hz) a | δH (J Hz) b | δH (J Hz) b | δH (J Hz) a | δH (J Hz) a | δH (J Hz) a | |
| 1 | - | - | - | - | - | - |
| 2 | - | - | - | - | - | - |
| 3 | 4.72, ddd (11.5, 9.2, 5.6) | - | - | - | 4.70, ddd (11.2, 9.2, 6.1) | 4.70, ddd (11.2, 9.2, 5.6) |
| 4 | 2.78, dd (12.8, 9.2) 1.90, dd (12.8, 11.5) | 7.39, s | 7.39, s | 7.37, s | 2.78, dd (12.8, 9.2) 1.90, dd (12.8, 11.2) | 2.78, dd (12.9, 9.2) 1.90, dd (12.9, 11.2) |
| 5 | - | - | - | - | - | - |
| 6 | 1.76, dq (14.7, 7.5) 1.68, dq (14.7, 7.5) | 1.76, q (7.5) | 1.76, q (7.4) | 1.82, q (7.4) | 1.77, dq (14.4, 7.5) 1.70, dq (14.4, 7.5) | 1.76, dq (14.7, 7.5) 1.69, dq (14.7, 7.5) |
| 7 | 1.0, t (7.5) | 0.76, t (7.5) | 0.76, t (7.4) | 0.90, t (7.4) | 1.00, t (7.5) | 1.00, t (7.5) |
| 8 | 1.47, s | 1.41, s | 1.41, s | 1.50, s | 1.49, s | 1.47, s |
| NH | 6.58, d (5.6) | 9.91, br s | 9.88, br s | 8.22, br s | 6.59, d (6.1) | 6.60, d (5.6) |
| 1′ | - | - | - | - | - | - |
| 2′ | 2.51, dd (15.4, 8.7) 2.45, dd (15.4, 3.2) | 2.60, dd (14.4, 8.0) 2.41, dd (14.4, 4.6) | 2.60, dd (14.4, 8.0) 2.41, dd (14.4, 5.2) | 2.63, dd (15.8, 8.7) 2.58, dd (15.8, 3.1) | 2.49, dd (15.5, 8.6) 2.45, dd (15.5, 3.2) | 2.49, dd (15.3, 8.5) 2.45, dd (15.3, 3.5) |
| 3′ | 5.01, br ddd (9.4, 8.7, 3.2) | 4.85, br dddd (9.2, 8.0, 4.6, 4.1) | 4.84, dddd (9.1, 8.0, 5.2, 4.7) | 5.06, ddt (9.4, 8.7, 3.1) | 5.01, m | 5.00, ddd (9.4, 8.5, 3.5) |
| OH | 5.16, br d (4.1) | 5.11, d (4.7) | ||||
| 4′ | 5.39, dd (11.3, 9.4) | 5.30, br dd (11.1, 9.2) | 5.28, dd (11.1, 9.1) | 5.41, dd (11.1, 9.4) | 5.35, dd (11.3, 9.4) | 5.33, br dd (11.3, 9.4) |
| 5′ | 6.07, br t (11.3) | 5.92, t (11.1) | 5.91, t (11.1) | 6.09, t (11.1) | 6.05, br t (11.3) | 6.05, br t (11.3) |
| 6′ | 6.47, br dd (15.0, 11.3) | 6.45, br dd (14.8, 11.1) | 6.41, br dd (14.8, 11.1) | 6.45, br dd (14.9, 11.1) | 6.34, br dd (14.9, 11.3,1.5) | 6.34, ddd (14.7, 11.3, 0.8) |
| 7′ | 5.82, br dt (15.0, 6.8) | 5.71, br dt (14.8, 7.1) | 5.71, br dt (14.8, 7.5, 7.2) | 5.81, dt (14.9, 7.2) | 5.75, dt (14.9, 6.7) | 5.75, br dt (14.7, 6.9) |
| 8′ | 2.36, br dd (6.8, 6.2) | 2.62, m 2.45, br dd (15.7, 8.3) | 2.32, br ddd (14.2, 7.2, 6.8) 2.28, br ddd (14.2, 7.5, 6.5) | 2.44, br dd (7.2, 6.8) | 2.87, br ddd (7.5, 6.7, 1.5) | 2.87, ddd (7.3, 6.9, 1.0) |
| 9′ | 3.01, dt (6.2, 4.2) | 3.95, br ddd (8.3, 4.4, 3.1) | 3.60, dddd (6.8, 6.5, 6.1, 3.1) | 3.72, ddd (7.2, 6.8, 3.5) | 5.37, ddd (10.9, 7.5, 1.5) | 5.38, ddd (10.4, 7.3, 0.8) |
| 9′-OH | - | - | 5.01, br d (6.1) | - | - | - |
| 10′ | 2.97, dt (5.9, 4.2) | 3.56, m | 3.91, ddd (9.8, 3.7, 3.1) | 3.91, ddd (9.8, 3.9, 3.5) | 5.47, ddd (10.9, 7.3, 1.5) | 5.48, ddd (10.4, 7.3, 1.0) |
| 10′-OH | - | 4.95, br d (5.6) | - | - | - | - |
| 11′ | 1.55, m | 1.43, m | 1.76, ddt (14.4, 7.1, 3.7) 1.66, ddt (14.1, 4.6, 9.8) | 1.82, m 1.66, ddt (14.1, 4.6, 9.8) | 2.04, m | 2.05, dt (14.7, 7.3) |
| 12′ | 1.44, m | 1.26, m | 1.45, m 1.33, m | 1.43, m 1.33, m | 1.39, m | 1.36, m |
| 13′ | 1.28–1.41 | 1.20–1.36 | 1.20–1.36 | 1.20–1.36 | 1.20–1.35 | 1.28–1.41 |
| 14′ | 1.28–1.41 | 1.20–1.36 | 1.20–1.36 | 1.20–1.36 | 1.20–1.35 | 1.28–1.41 |
| 15′ | 1.28–1.41 | 1.20–1.36 | 1.20–1.36 | 1.20–1.36 | 1.24, m | 1.28–1.41 |
| 16′ | 1.30, m | 1.25, m | 1.24, m | 1.26, m | 1.32, m | 1.30, m |
| 17′ | 1.32, m | 1.27, m | 1.27, m | 1.28, m | 0.89, t (7.0) | 1.30, m |
| 18′ | 0.89, t (7.0) | 0.86, t (6.9) | 0.86, t (6.8) | 0.88, t (7.2) | - | 0.89, t (6.9) |

2.2. Antibacterial, Antifungal and Antiprotozoal Activities of Bacterial-Derived Metabolites
| Strains (Growth media; temperature °C) | Bromopyrroles | Korormicins G–I and K | |||||
|---|---|---|---|---|---|---|---|
| BAC-A | TBP | 1 | 2 | 3 | 4 | 6 | |
| Pseudoalteromonas haloplanctis (MA; 28) | n | y | y | y | y | y | y |
| Pseudoalteromonas piscicida (MA; 28) | n | y | y | y | y | y | y |
| Pseudoalteromonas undina (MA; 28) | n | y | y | y | y | y | y |
| Pseudoalteromonas strain. J010 (MA; 28) | n | y | n | n | n | n | n |
| Pseudomonas aeruginosa (LB10; 37) | n | y | y | y | y | y | y |
| Vibrio campbellii (MA; 28) | n | y | y | y | y | y | y |
| Vibrio vulnificus (LB10; 37) | n | y | y | y | y | y | y |
| Vibrio coralliilyticus (MA; 28) | n | y | y | y | y | y | y |
| Vibrio harveyi (MA; 28) | n | y | y | y | y | y | y |
| Shewanella aquimarina (MA; 28) | n | y | y | y | y | y | y |
| Staphylococcus aureus (LB10; 37) | n | y | y | n | n | n | n |
| Candida albicans (MA; 28) | n | y | n | n | n | n | n |
| Tetrahymena pyriformis (NSS; ambient) | y | y | n | n | n | n | n |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Isolation of Bacterial Metabolites
3.2.1. 4′-((3,4,5-Tribromo-1H-pyrrol-2-yl)methyl)phenol (1)
3.2.2. (3S*, 5S*, 3′R*, 4′Z, 6′E, 9′S*, 10′R*) Korormicin G (2)
3.2.3. (3S*, 3′R*, 4′Z, 6′E) Korormicin H (3)
3.2.4. (3S*, 3′R*, 4′Z, 6′E) Korormicin I (4)
3.2.5. (3S*, 5S*, 3′R*, 4′Z, 6′E, 9′Z*) Korormicin J (5)
3.2.6. (3S*, 5S*, 3′R*, 4′Z, 6′E, 9′Z*) Korormicin K (6)
3.3. Antibacterial, Antifungal and Antiprotozoal Bioassays
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hay, M.E. Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 193–212. [Google Scholar] [CrossRef]
- Hadfield, M.G. Biofilms and marine invertebrate larvae: What bacteria produce that larvae use to choose settlement sites. Annu. Rev. Mar. Sci. 2011, 3, 453–470. [Google Scholar] [CrossRef]
- Tebben, J.; Tapiolas, D.M.; Motti, C.A.; Abrego, D.; Negri, A.P.; Blackall, L.L.; Steinberg, P.D.; Harder, T. Induction of larval metamorphosis of the coral Acropora millepora by tetrabromopyrrole isolated from a Pseudoalteromonas bacterium. PLoS One 2011, 6, e19082. [Google Scholar] [CrossRef]
- Andersen, R.J.; Wolfe, M.S.; Faulkner, D.J. Autotoxic antibiotic production by a marine Chromobacterium. Mar. Biol. 1974, 27, 281–285. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Takadera, T.; Adachi, K.; Nishijima, M.; Sano, H. Korormicin, a novel antibiotic specifically active against marine Gram-negative bacteria, produced by a marine bacterium. J. Antibiot. 1997, 50, 949–953. [Google Scholar] [CrossRef]
- Fehér, D.; Barlow, R.; Mcatee, J.; Hemscheidt, T.K. Highly brominated antimicrobial metabolites from a marine Pseudoalteromonas sp. J. Nat. Prod. 2010, 73, 1963–1966. [Google Scholar] [CrossRef]
- Bowman, J.P. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Mar. Drugs 2007, 5, 220–241. [Google Scholar] [CrossRef]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The seaweed holobiont: Understanding bacteria-seaweed interactions. FEMS Microbiol. Rev. 2013, 37, 462–476. [Google Scholar] [CrossRef]
- Egan, S.; Thomas, T.; Kjelleberg, S. Unlocking the diversity and biotechnological potential of marine surface associated microbial communities. Curr. Opin. Microbiol. 2008, 11, 219–225. [Google Scholar] [CrossRef]
- Engel, S.; Jensen, P.R.; Fenical, W. Chemical ecology of marine microbial defense. J. Chem. Ecol. 2002, 28, 1971–1985. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Adachi, K.; Nishida, F.; Mochida, K. Planar structure and antibacterial activity of korormicin derivatives isolated from Pseudoalteromonas sp. F-420. J. Antibiot. 2003, 56, 866–870. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yoshida, S.; Nakayama, Y. Total synthesis of korormicin. Eur. J. Org. Chem. 2001, 2001, 1873–1881. [Google Scholar] [CrossRef]
- Speitling, M.; Smetanina, O.F.; Kuznetsova, T.A.; Laatsch, H. Bromoalterochromides A and A′, unprecedented chromopeptides from a marine Pseudoalteromonas maricaloris strain KMM 636T. J. Antibiot. 2007, 60, 36–42. [Google Scholar] [CrossRef]
- John, E.A.; Pollet, P.; Gelbaum, L.; Kubanek, J. Regioselective syntheses of 2,3,4-tribromopyrrole and 2,3,5-tribromopyrrole. J. Nat. Prod. 2004, 67, 1929–1931. [Google Scholar] [CrossRef]
- Schwalm, C.S.; Castro, I.B.D.D.; Ferrari, J.; Oliveira, F.L.D.; Aparicio, R.; Correia, C.R.D. Synthesis of pentabromopseudilin and other arylpyrrole derivatives via Heck arylations. Tetrahedron Lett. 2012, 53, 1660–1663. [Google Scholar] [CrossRef]
- Watson, W.T.; Minogue, T.D.; von Bodman, S.B.; Val, D.L.; Churchill, M.E.A. Structural basis and specificity of acyl-homoserine lactone signal production in bacterial quorum sensing. Mol. Cell 2004, 9, 685–694. [Google Scholar]
- Hayashi, M.; Shibata, N.; Nakayama, Y.; Yoshikawa, K.; Unemotoa, T. Korormicin insensitivity in Vibrio alginolyticus is correlated with a single point mutation of Gly-140 in the NqrB subunit of the Na+-translocating NADH-quinone reductase. Arch. Biochem. Biophys. 2002, 401, 173–177. [Google Scholar] [CrossRef]
- Tapiolas, D.M.; Bowden, B.F.; Abou-Mansour, E.; Willis, R.H.; Doyle, J.R.; Muirhead, A.N.; Liptrot, C.; Llewellyn, L.E.; Wolff, C.W.W.; Wright, A.D.; et al. Eusynstyelamides A, B, and C, nNOS inhibitors, from the ascidian Eusynstyela latericius. J. Nat. Prod. 2009, 72, 1115–1120. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic sensitivity testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar]
- Matz, C.; Webb, J.S.; Schupp, P.J.; Phang, S.Y.; Penesyan, A.; Egan, S.; Steinberg, P.; Kjelleberg, S. Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. PLoS One 2008, 3, e2744. [Google Scholar] [CrossRef]
- Vaatanen, P. Microbiological studies in coastal waters of the Northern Baltic sea, I. Distribution and abundance of bacteria and yeasts in the Tvärminne area. In The Walter and Andree De Nottbeck Foundation Scientific Report; Preliminary report for 1973-74, ISBN 951-95341-0-5; University of Helsinki: Hanko, Finland, 1976; Volume 1, pp. 1–58. [Google Scholar]
- Penesyan, A.; Marshall-Jones, Z.; Holmström, C.; Kjelleberg, S.; Egan, S. Antimicrobial activity observed among cultured marine epiphytic bacteria reflects their potential as a source of new drugs. FEMS Microbiol. Ecol. 2009, 69, 113–124. [Google Scholar] [CrossRef]
- Dobretsov, S.; Dahms, H.-U.; Qian, P.-Y. Inhibition of biofouling by marine microorganisms and their metabolites. Biofouling 2006, 22, 43–54. [Google Scholar] [CrossRef]
- Wiese, J.; Thiel, V.; Nagel, K.; Staufenberger, T.; Imhoff, J. Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the Baltic Sea. Mar. Biotechnol. 2009, 11, 287–300. [Google Scholar] [CrossRef] [Green Version]
- Egan, S.; Thomas, T.; Holmstrom, C.; Kjelleberg, S. Phylogenetic relationship and antifouling activity of bacterial epiphytes from the marine alga Ulva lactuca. Environ. Microbiol. 2000, 2, 343–347. [Google Scholar] [CrossRef]
- Wagner-Döbler, I.; Thiel, V.; Eberl, L.; Allgaier, M.; Bodor, A.; Meyer, S.; Ebner, S.; Hennig, A.; Pukall, R.; Schulz, S. Discovery of complex mixtures of novel long-chain quorum sensing signals in free-living and host-associated marine Alphaproteobacteria. ChemBioChem 2005, 6, 2195–2206. [Google Scholar] [CrossRef]
- Haber, M.; Carbone, M.; Mollo, E.; Gavagnin, M.; Ilan, M. Chemical defense against predators and bacterial fouling in the Mediterranean sponges Axinella polypoides and A. verrucosa. Mar. Ecol. Prog. Ser. 2011, 422, 113–122. [Google Scholar] [CrossRef]
- Scala, F.; Fattorusso, E.; Menna, M.; Taglialatela-Scafati, O.; Tierney, M.; Kaiser, M.; Tasdemir, D. Bromopyrrole alkaloids as lead compounds against protozoan parasites. Mar. Drugs 2010, 8, 2162–2174. [Google Scholar] [CrossRef]
- Xiong, S.; Pang, H.-D. In vitro and in vivo antineoplastic activity of a novel bromopyrrole and its potential mechanism of action. Br. J. Pharmacol. 2010, 159, 909–918. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tebben, J.; Motti, C.; Tapiolas, D.; Thomas-Hall, P.; Harder, T. A Coralline Algal-Associated Bacterium, Pseudoalteromonas Strain J010, Yields Five New Korormicins and a Bromopyrrole. Mar. Drugs 2014, 12, 2802-2815. https://doi.org/10.3390/md12052802
Tebben J, Motti C, Tapiolas D, Thomas-Hall P, Harder T. A Coralline Algal-Associated Bacterium, Pseudoalteromonas Strain J010, Yields Five New Korormicins and a Bromopyrrole. Marine Drugs. 2014; 12(5):2802-2815. https://doi.org/10.3390/md12052802
Chicago/Turabian StyleTebben, Jan, Cherie Motti, Dianne Tapiolas, Peter Thomas-Hall, and Tilmann Harder. 2014. "A Coralline Algal-Associated Bacterium, Pseudoalteromonas Strain J010, Yields Five New Korormicins and a Bromopyrrole" Marine Drugs 12, no. 5: 2802-2815. https://doi.org/10.3390/md12052802