Structural Elucidation of Novel Saponins in the Sea Cucumber Holothuria lessoni
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Saponins by ESI-MS

| [M + Na]+ m/z | MW | Formula | Compound’s Name | Novel (N)/ Published(P) | Sea Cucumber Species A. = Actinopyga; B. = Bohadschia; P. = Pearsonothuria; H. = Holothuria | References |
|---|---|---|---|---|---|---|
| 1071.6 | 1048 | C47H93NaO21S | Unidentified | N | H. lessoni | [12] |
| 1083.3 | 1060 | C58H64O25 | Unidentified | N | H. lessoni | [12] |
| 1087.6 | 1064 | C47H93NaO22S | Unidentified | N | H. lessoni | [12] |
| 1123.5 | 1100 | C54H84O23 | Unidentified | N | H. lessoni | [12] |
| 1125.5 | 1102 | C54H86O23 | Holothurinoside C | P | H. lessoni, H. forskali, A. agassizi, H. scabra, H. fuscocinerea and H. impatiens | [12,67,69,77,89,90] |
| Holothurinoside C1 | P | |||||
| 1127.6 | 1104 | C54H88O23 | Unidentified | N | H. lessoni | - |
| Unidentified | N | H. lessoni | - | |||
| Unidentified | N | H. lessoni | - | |||
| 1141.5 | 1118 | C54H86O24 | Desholothurin A | P | H. lessoni, H. forskali, H. nobilis, A. agassizi, B. argus, B. cousteaui, H. leucospilota, P. graeffei | [4,12,13,77,89,90,91,92,93] |
| (Nobiliside 2a), | P | |||||
| Desholothurin A1 (Arguside E) | P | |||||
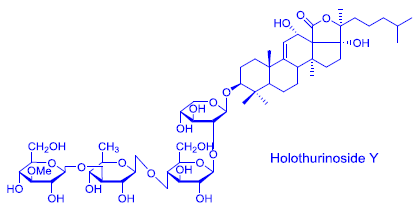
| 1149.2 | 1126 | a * | Holothurinoside T | P | H. lessoni | [12] |
| 1157.5 | 1134 | C54H109O25 | Holothurinoside J1 | P | H. lessoni, B. subrubra | [12,59] |
| C49H91NaO25S | Unidentified | N | H. lessoni | - | ||
| 1169.5 | 1170 | C55H87NaO23S | Unidentified | N | H. lessoni | - |
| 1227.4 | 1204 | C54H85NaO26S | Fuscocinerosides B/C, Scabraside A or 24–dehydroechinoside A, Unidentified | P | B. subrubra, H. lessoni, H. scabra, H. leucospilota, H. fuscocinerea, A. agassizi, and H. impatiens, P. graeffei, A. echinites | [12,13,36,64,67,69,90,94,95,96] |
| P | ||||||
| N | ||||||
| 1243.5 | 1220 | C54H85NaO27S | Holothurin A Scabraside B 17-Hydroxy fuscocineroside B 25-Hydroxy fuscocinerosiden B | P P P P | H. lessoni, H. scabra, H. atra, H. leucospilota, H. arenicola, H. cinerascens, H. coluber, H. cubana, H. difficilis, H. gracilis, H. pervicax, H. lubrica, H. polii, H. pulla, H. squamifera, H. surinamensis, H. tubulosa, P. graeffei, A. agassizi, A. echinites, A. lecanora, A. mauritana, H. grisea, H. hilla, H. Mexicana, H. moebi, H. nobilis, H. monacaria, H. forskali, H. edulis, H. axiloga, H. fuscocinerea and H. impatiens | [12,36,44,67,72,79,93,96,97,98,99,100,101,102,103] |
| 1259.5 | 1236 | C54H85NaO28S | Holothurin A3 | P | H. lessoni, H. scabra, H. fuscocinerea and H. impatiens | [12,44,69] |
| Unidentified | N | H. lessoni | - | |||
| 1287.6 | 1264 | C60H96O28 | Holothurinoside E, | P | H. lessoni, H. forskali | [12,77] |
| Holothurinoside E1 | P | H. lessoni, H. forskali | [12,77] | |||
| Holothurinoside O | P | H. lessoni | [12] | |||
| Holothurinoside P | P | H. lessoni | [12] | |||
| 17-dehydroxyholothurinoside A | P | H. lessoni, H. grisea, B. cousteaui | [4,12,104] | |||
| 1301.6 | 1278 | C61H98O28 | Holothurinoside M | P | H. lessoni, H. forskali, H. scabra, H. fuscocinerea and H. impatiens H. lessoni | [12,67,69,70] |
| C60H94O29 | Unidentified | N | - | |||
| 1303.6 | 1280 | C60H96O29 | Holothurinoside A | P | H. lessoni, H. forskali, B. vitiensis, B. cousteaui | [4,12,67,77,89] |
| Holothurinoside A1 | P | H. lessoni, H. forskali, B. vitiensis, B. cousteaui | [4,12,67,77,89] | |||
| Holothurinoside Q | P | H. lessoni | [12] | |||
| Holothurinoside S | P | H. lessoni | [12] | |||
| Holothurinoside R | P | H. lessoni | [12] | |||
| Holothurinoside R1 | P | H. lessoni | [12] | |||
| 1317.6 | 1294 | C61H98O29 | Holothurinoside N | P | H. lessoni, H. forskali | [12,67] |
| 1475.6 | 1452 | C65H96O36 | Unidentified | N | H. lessoni | - |
| 1477.7 | 1454 | C61H114O38 | Unidentified | N | H. lessoni | - |
| 1479.7 | 1456 | C67H108O34 | Holothurinoside I | P | H. lessoni, H. forskali | [12,92] |
| 1495.7 | 1472 | C61H116O39 | Holothurinoside K1 | P | B. subrubra, H. lessoni | [12,59] |
| C72H112O31 | Unidentified | N | H. lessoni | - |
2.1.1. Determination of the Saponin Structures by ESI-MS/MS


| Diagnostic Ions in CID Spectra of [M + Na]+ | ||||
|---|---|---|---|---|
| m/z signals (Da) | ||||
| 493 | 507 | 523 | 657 | |
| Chemical signatures | MeGlc-Glc-Xyl + Na | MeGlc-Glc-Qui + Na | MeGlc-Glc-Glc +Na | MeGlc-Glc-Qui-Xyl + Na |






2.2. Key Diagnostic Fragments in the Sea Cucumber Saponins

2.3. MALDI-MS/MS Analysis of Saponins in Positive Ion Mode


3. Experimental Section
3.1. Sea Cucumber Sample
3.2. Extraction Protocol
3.2.1. Extraction of Saponins
3.3. Purification of the Extract
3.4. Thin Layer Chromatography (TLC)
3.5. High Performance Centrifugal Partition Chromatography (HPCPC or CPC)
3.6. Mass Spectrometry
3.6.1. MALDI MS
3.6.2. ESI MS
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Purcell, S.W.; Samyn, Y.; Conand, C. Commercially Important Sea Cucumbers of the World; FAO Species Catalogue for Fishery Purposes. No. 6; FAO: Rome, Italy, 2012; p. 150. [Google Scholar]
- Waller, G.R.; Yamasaki, K. Saponins Used in Food and Agriculture; Plenum Press: New York, NY, USA, 1996; Volume 405. [Google Scholar]
- Hostettmann, K.; Marston, A. Saponins; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Elbandy, M.; Rho, J.; Afifi, R. Analysis of saponins as bioactive zoochemicals from the marine functional food sea cucumber Bohadschia cousteaui. Eur. Food Res. Technol. 2014, 238, 1–19. [Google Scholar] [CrossRef]
- Venugopal, V. Marine Products for Healthcare: Functional and Bioactive Nutraceutical Compounds from the Ocean; CRC Press Taylor & Francis Group: New York, NY, USA, 2009; Volume 13. [Google Scholar]
- Ridzwan, B.H. Sea Cucumbers, a Malaysian Heritage, 1st ed.; Research Centre of International Islamic University Malaysia (IIUM), Kuala Lumpur Wilayah Persekutuan: Kuala Lumpur, Malaysia, 2007; pp. 1–15, 89–128. [Google Scholar]
- Toral-Granda, V. The Biological and Trade Status of Sea Cucumbers in the Families Holothuriidae and Stichopodidae; Convention on International Trade in Endangered Species of Wild Flora and Fauna: Hague, The Netherlands, 2006. [Google Scholar]
- Zhong, Y.; Khan, M.A.; Shahidi, F. Compositional characteristics and antioxidant properties of fresh and processed sea cucumber Cucumaria frondosa. J. Agric. Food Chem. 2007, 55, 1188–1192. [Google Scholar] [CrossRef]
- Kiew, P.L.; Don, M.M. Jewel of the seabed: Sea cucumbers as nutritional and drug candidates. Int. J. Food Sci. Nutr. 2012, 63, 616–136. [Google Scholar]
- Lovatelli, A.; Conand, C. Advances in Sea Cucumber Aquaculture and Management; FAO: Rome, Italy, 2004. [Google Scholar]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–805. [Google Scholar] [CrossRef]
- Bahrami, Y.; Zhang, W.; Franco, C. Discovery of novel saponins from the viscera of the sea cucumber Holothuria lessoni. Mar. Drugs 2014, 12, 2633–2667. [Google Scholar] [CrossRef]
- Caulier, G.; Van Dyck, S.; Gerbaux, P.; Eeckhaut, I.; Flammang, P. Review of saponin diversity in sea cucumbers belonging to the family Holothuriidae. SPC Beche-de-mer Inf. Bull. 2011, 31, 48–54. [Google Scholar]
- Dong, P.; Xue, C.; Du, Q. Separation of two main triterpene glycosides from sea cucumber Pearsonothuria graeffei by high-speed countercurrent chromatography. Acta Chromatogr. 2008, 20, 269–276. [Google Scholar] [CrossRef]
- Han, H.; Zhang, W.; Yi, Y.H.; Liu, B.S.; Pan, M.X.; Wang, X.H. A novel sulfated holostane glycoside from sea cucumber Holothuria leucospilota. Chem. Biodivers. 2010, 7, 1764–1769. [Google Scholar] [CrossRef]
- Naidu, A.S. Natural Food Antimicrobial Systems; CRC Press: New York, NY, USA, 2000. [Google Scholar]
- Zhang, S.L.; Li, L.; Yi, Y.H.; Sun, P. Philinopsides E and F, two new sulfated triterpene glycosides from the sea cucumber Pentacta quadrangularis. Nat. Prod. Res. 2006, 20, 399–407. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Yi, Y.H.; Tang, H.F.; Li, L.; Sun, P.; Wu, J. Two new bioactive triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. J. Asian Nat. Prod. Res. 2006, 8, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, L.; Yi, Y.H.; Zou, Z.R.; Sun, P. Philinopgenin A, B, and C, three new triterpenoid aglycones from the sea cucumber Pentacta quadrangulasis. Mar. Drugs 2004, 2, 185–191. [Google Scholar] [CrossRef]
- Chludil, H.D.; Muniain, C.C.; Seldes, A.M.; Maier, M.S. Cytotoxic and antifungal triterpene glycosides from the Patagonian sea cucumber Hemoiedema spectabilis. J. Nat. Prod. 2002, 65, 860–865. [Google Scholar] [CrossRef]
- Maier, M.S.; Roccatagliata, A.J.; Kuriss, A.; Chludil, H.; Seldes, A.M.; Pujol, C.A.; Damonte, E.B. Two new cytotoxic and virucidal trisulfated triterpene glycosides from the Antarctic sea cucumber Staurocucumis liouvillei. J. Nat. Prod. 2001, 64, 732–736. [Google Scholar] [CrossRef]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The saponins–polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; He, J.; Xia, M.; Xu, L.; Yang, S. Structure analysis of triterpene saponins in Polygala tenuifolia by electrospray ionization ion trap multiple-stage mass spectrometry. J. Mass Spectrom. 2007, 42, 861–873. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides from sea cucucmbers (Holothurioidea, Echinodermata). Biological activities and functions. Stud. Nat. Prod. Chem. 2008, 35, 135–196. [Google Scholar] [CrossRef]
- Jha, R.K.; Zi-rong, X. Biomedical Compounds from Marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar] [CrossRef]
- Friess, S.; Standaert, F.; Whitcomb, E.; Nigrelli, R.; Chanley, J.; Sobotka, H. Some pharmacologic properties of holothurin, an active neurotoxin from the sea cucumber. J. Pharmacol. Exp. Ther. 1959, 126, 323–329. [Google Scholar]
- Kim, S.K.; Himaya, S.W.; kang, K.H. Sea cucumber saponins realization of their anticancer effects. In Marine Pharmacognosy: Trends and Applications; Kim, S.K., Ed.; CRC Press: New York, NY, USA, 2012; pp. 119–128. [Google Scholar]
- Aminin, D.L.; Pislyagin, E.A.; Menchinskaya, E.S.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Immunomodulatory and anticancer activity of sea cucumber triterpene glycosides. Stud. Nat. Prod. Chem. 2014, 41, 75–94. [Google Scholar] [CrossRef]
- Sarhadizadeh, N.; Afkhami, M.; Ehsanpour, M. Evaluation bioactivity of a sea cucumber, Stichopus hermanni from Persian Gulf. Eur. J. Exp. Biol. 2014, 4, 254–258. [Google Scholar]
- Mokhlesi, A.; Saeidnia, S.; Gohari, A.R.; Shahverdi, A.R.; Nasrolahi, A.; Farahani, F.; Khoshnood, R.; Es’ Haghi, N. Biological activities of the sea cucumber Holothuria leucospilota. Asian J. Anim. Vet. Adv. 2012, 7, 243–249. [Google Scholar] [CrossRef]
- Mohammadizadeh, F.; Ehsanpor, M.; Afkhami, M.; Mokhlesi, A.; Khazaali, A.; Montazeri, S. Antibacterial, antifungal and cytotoxic effects of a sea cucumber Holothuria leucospilota, from the north coast of the Persian Gulf. J. Mar. Biol. Assoc. UK 2013, 93, 1401–1405. [Google Scholar] [CrossRef]
- Mohammadizadeh, F.; Ehsanpor, M.; Afkhami, M.; Mokhlesi, A.; Khazaali, A.; Montazeri, S. Evaluation of antibacterial, antifungal and cytotoxic effects of Holothuria scabra from the north coast of the Persian Gulf. J. Med. Mycol. 2013, 23, 225–229. [Google Scholar] [CrossRef]
- Yamanouchi, T. On the poisonous substance contained in holothurians. Publ. Seto Mar. Biol. Lab. 1955, 4, 183–203. [Google Scholar]
- Avilov, S.A.; Drozdova, O.A.; Kalinin, V.I.; Kalinovsky, A.I.; Stonik, V.A.; Gudimova, E.N.; Riguera, R.; Jimenez, C. Frondoside C, a new nonholostane triterpene glycoside from the sea cucumber Cucumaria frondosa: Structure and cytotoxicity of its desulfated derivative. Can. J. Chem. 1998, 76, 137–141. [Google Scholar]
- Han, H.; Yi, Y.; Xu, Q.; La, M.; Zhang, H. Two new cytotoxic triterpene glycosides from the sea cucumber Holothuria scabra. Planta Med. 2009, 75, 1608–1612. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Avilov, S.A.; Kalinina, E.Y.; Korolkova, O.G.; Kalinovsky, A.I.; Stonik, V.A.; Riguera, R.; Jimenez, C. Structure of eximisoside A, a novel triterpene glycoside from the Far-Eastern sea cucumber Psolus eximius. J. Nat. Prod. 1997, 60, 817–819. [Google Scholar] [CrossRef]
- Kitagawa, I.; Yamanaka, H.; Kobayashi, M.; Nishino, T.; Yosioka, I.; Sugawara, T. Saponin and sapogenol. XXVII. Revised structures of holotoxin A and holotoxin B, two antifungal oligoglycosides from the sea cucumber Stichopus japonicus Selenka. Chem. Pharm. Bull. (Tokyo) 1978, 26, 3722–3731. [Google Scholar] [CrossRef]
- Miyamoto, T.; Togawa, K.; Higuchi, R.; Komori, T.; Sasaki, T. Structures of four new triterpenoid oligoglycosides: DS-penaustrosides A, B, C, and D from the sea cucumber Pentacta australis. J. Nat. Prod. 1992, 55, 940–946. [Google Scholar] [CrossRef]
- Liu, B.S.; Yi, Y.H.; Li, L.; Sun, P.; Yuan, W.H.; Sun, G.Q.; Han, H.; Xue, M. Argusides B and C, two new cytotoxic triterpene glycosides from the sea cucumber Bohadschia argus Jaeger. Chem. Biodivers. 2008, 5, 1288–1297. [Google Scholar] [CrossRef]
- Girard, M.; Bélanger, J.; ApSimon, J.W.; Garneau, F.X.; Harvey, C.; Brisson, J.R. Frondoside A. A novel triterpene glycoside from the holothurian Cucumaria frondosa. Can. J. Chem. 1990, 68, 11–18. [Google Scholar] [CrossRef]
- Thompson, J.; Walker, R.; Faulkner, D. Screening and bioassays for biologically-active substances from forty marine sponge species from San Diego, California, USA. Mar. Biol. 1985, 88, 11–21. [Google Scholar] [CrossRef]
- Campagnuolo, C.; Fattorusso, E.; Taglialatela-Scafati, O. Feroxosides A-B, two norlanostane tetraglycosides from the Caribbean sponge Ectyoplasia ferox. Tetrahedron 2001, 57, 4049–4055. [Google Scholar] [CrossRef]
- Dang, N.H.; Thanh, N.V.; Kiem, P.V.; Huong le, M.; Minh, C.V.; Kim, Y.H. Two new triterpene glycosides from the Vietnamese sea cucumber Holothuria scabra. Arch. Pharm. Res. 2007, 30, 1387–1391. [Google Scholar] [CrossRef]
- Kerr, R.G.; Chen, Z. In vivo and in vitro biosynthesis of saponins in sea cucumbers. J. Nat. Prod. 1995, 58, 172–176. [Google Scholar] [CrossRef]
- Kim, S.K.; Himaya, S.W. Triterpene glycosides from sea cucumbers and their biological activities. Adv. Food Nutr. Res. 2012, 65, 297–319. [Google Scholar] [CrossRef]
- Stonik, V.A.; Kalinin, V.I.; Avilov, S.A. Toxins from sea cucumbers (holothuroids): Chemical structures, properties, taxonomic distribution, biosynthesis and evolution. J. Nat. Toxins 1999, 8, 235–248. [Google Scholar]
- Chludil, H.D.; Murray, A.P.; Seldes, A.M.; Maier, M.S. Biologically active triterpene glycosides from sea cucumbers (Holothuroidea, Echinodermata). Stud. Nat. Prod. Chem. 2003, 28, 587–615. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Smirnov, A.V. Sea cucumbers triterpene glycosides, the recent progress in structural elucidation and chemotaxonomy. Phytochem. Rev. 2005, 4, 221–236. [Google Scholar] [CrossRef]
- Habermehl, G.; Volkwein, G. Aglycones of the toxins from the Cuvierian organs of Holothuria forskali and a new nomenclature for the aglycones from Holothurioideae. Toxicon 1971, 9, 319–326. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Tang, H.F.; Yi, Y.H. Cytotoxic triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. Fitoterapia 2007, 78, 283–287. [Google Scholar] [CrossRef]
- Stonik, V.A.; Elyakov, G.B. Secondary metabolites from echinoderms as chemotaxonomic markers. Bioorg. Mar. Chem. 1988, 2, 43–86. [Google Scholar] [CrossRef]
- Antonov, A.S.; Avilov, S.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Kalinin, V.I.; Taboada, S.; Ballesteros, M.; Avila, C. Triterpene glycosides from Antarctic sea cucumbers III. Structures of liouvillosides A4 and A5, two minor disulphated tetraosides containing 3-O-methylquinovose as terminal monosaccharide units from the sea cucumber Staurocucumis liouvillei (Vaney). Nat. Prod. Res. 2011, 25, 1324–1333. [Google Scholar] [CrossRef]
- Avilov, S.A.; Silchenko, A.S.; Antonov, A.S.; Kalinin, V.I.; Kalinovsky, A.I.; Smirnov, A.V.; Dmitrenok, P.S.; Evtushenko, E.V.; Fedorov, S.N.; Savina, A.S.; Shubina, L.K.; Stonik, V.A. Synaptosides A and A1, triterpene glycosides from the sea cucumber Synapta maculata containing 3-O-methylglucuronic acid and their cytotoxic activity against tumor cells. J. Nat. Prod. 2008, 71, 525–531. [Google Scholar] [CrossRef]
- Antonov, A.S.; Avilov, S.A.; Kalinovsky, A.I.; Anastyuk, S.D.; Dmitrenok, P.S.; Evtushenko, E.V.; Kalinin, V.I.; Smirnov, A.V.; Taboada, S.; Ballesteros, M.; et al. Triterpene glycosides from antarctic sea cucumbers. 1. structure of liouvillosides A1, A2, A3, B1, and B2 from the sea cucumber Staurocucumis liouvillei: New procedure for separation of highly polar glycoside fractions and taxonomic revision. J. Nat. Prod. 2008, 71, 1677–1685. [Google Scholar] [CrossRef]
- Aminin, D.L.; Chaykina, E.L.; Agafonova, I.G.; Avilov, S.A.; Kalinin, V.I.; Stonik, V.A. Antitumor activity of the immunomodulatory lead Cumaside. Int. Immunopharmacol. 2010, 10, 648–654. [Google Scholar] [CrossRef]
- Iniguez-Martinez, A.M.; Guerra-Rivas, G.; Rios, T.; Quijano, L. Triterpenoid oligoglycosides from the sea cucumber Stichopus parvimensis. J. Nat. Prod. 2005, 68, 1669–1673. [Google Scholar] [CrossRef]
- Xu, R.; Ye, Y.; Zhao, W. Saponins. In Introduction to Natural Products Chemistry; CRC Press: Boca Raton, FL, USA, 2012; pp. 125–145. [Google Scholar]
- Van Dyck, S.; Gerbaux, P.; Flammang, P. Qualitative and quantitative saponin contents in five sea cucumbers from the Indian Ocean. Mar. Drugs 2010, 8, 173–189. [Google Scholar] [CrossRef]
- Avilov, S.A.; Antonov, A.S.; Drozdova, O.A.; Kalinin, V.I.; Kalinovsky, A.I.; Stonik, V.A.; Riguera, R.; Lenis, L.A.; Jiménez, C. Triterpene glycosides from the far-eastern sea cucumber Pentamera calcigera. 1. Monosulfated glycosides and cytotoxicity of their unsulfated derivatives. J. Nat. Prod. 2000, 63, 65–71. [Google Scholar] [CrossRef]
- Avilov, S.A.; Kalinovsky, A.I.; Kalinin, V.I.; Stonik, V.A.; Riguera, R.; Jiménez, C. Koreoside A, a new nonholostane triterpene glycoside from the sea cucumber Cucumaria koraiensis. J. Nat. Prod. 1997, 60, 808–810. [Google Scholar] [CrossRef]
- Avilov, S.A.; Antonov, A.S.; Drozdova, O.A.; Kalinin, V.I.; Kalinovsky, A.I.; Riguera, R.; Lenis, L.A.; Jimenez, C. Triterpene glycosides from the far eastern sea cucumber Pentamera calcigera II: Disulfated glycosides. J. Nat. Prod. 2000, 63, 1349–1355. [Google Scholar] [CrossRef]
- Avilov, S.A.; Antonov, A.S.; Silchenko, A.S.; Kalinin, V.I.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A.; Riguera, R.; Jimenez, C. Triterpene glycosides from the far eastern sea cucumber Cucumaria conicospermium. J. Nat. Prod. 2003, 66, 910–916. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Yi, Y.H.; Tang, H.F. Bioactive triterpene glycosides from the sea cucumber Holothuria fuscocinerea. J. Nat. Prod. 2006, 69, 1492–1495. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Yi, Y.H.; Tang, H.F. Cytotoxic sulfated triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. Chem. Biodivers. 2006, 3, 807–817. [Google Scholar] [CrossRef]
- Jia, L.; Qian, K. An evidence-based perspective of panax ginseng (Asian Ginseng) and panax quinquefolius (American Ginseng) as a preventing or supplementary therapy for cancer patients. In Evidence-based Anticancer Materia Medica; Springer Verlag: New York, NY, USA, 2011; pp. 85–96. [Google Scholar]
- Caulier, G.; Flammang, P.; Gerbaux, P.; Eeckhaut, I. When a repellent becomes an attractant: Harmful saponins are kairomones attracting the symbiotic Harlequin crab. Sci. Rep. 2013, 3, 1–5. [Google Scholar]
- Bakus, G.J. Defensive mechanisms and ecology of some tropical holothurians. Mar. Biol. 1968, 2, 23–32. [Google Scholar] [CrossRef]
- Bondoc, K.G.V.; Lee, H.; Cruz, L.J.; Lebrilla, C.B.; Juinio-Meñez, M.A. Chemical fingerprinting and phylogenetic mapping of saponin congeners from three tropical holothurian sea cucumbers. Comp Biochem. Physiol. B 2013, 166, 182–193. [Google Scholar] [CrossRef]
- Van Dyck, S.; Caulier, G.; Todesco, M.; Gerbaux, P.; Fournier, I.; Wisztorski, M.; Flammang, P. The triterpene glycosides of Holothuria forskali: Usefulness and efficiency as a chemical defense mechanism against predatory fish. J. Exp. Biol. 2011, 214, 1347–1356. [Google Scholar] [CrossRef]
- Kalyani, G.A.; Kakrani, H.K.N.; Hukkeri, V.I. Holothurin-A Review. Indian J. Nat. Prod. 1988, 4, 3–8. [Google Scholar]
- Kobayashi, M.; Hori, M.; Kan, K.; Yasuzawa, T.; Matsui, M.; Suzuki, S.; Kitagawa, I. Marine natural products. XXVII: Distribution of lanostane-type triterpene oligoglycosides in ten kinds of Okinawan Sea cucumbers. Chem. Pharm. Bull. 1991, 39, 2282–2287. [Google Scholar] [CrossRef]
- Kalinin, V.; Anisimov, M.; Prokofieva, N.; Avilov, S.; Afiyatullov, S.S.; Stonik, V. Biological activities and biological role of triterpene glycosides from holothuroids (Echinodermata). Echinoderm Stud. 1996, 5, 139–181. [Google Scholar]
- aKalinin, V.I.; Prokofieva, N.G.; Likhatskaya, G.N.; Schentsova, E.B.; Agafonova, I.G.; Avilov, S.A.; Drozdova, O.A. Hemolytic activities of triterpene glycosides from the holothurian order Dendrochirotida: Some trends in the evolution of this group of toxins. Toxicon 1996, 34, 475–483. [Google Scholar] [CrossRef]
- Mercier, A.; Sims, D.W.; Hamel, J.F. Advances in Marine Biology: Endogenous and Exogenous Control of Gametogenesis and Spawning in Echinoderms; Academic Press: New York, NY, USA, 2009; Volume 55. [Google Scholar]
- Massin, C.; Uthicke, S.; Purcell, S.W.; Rowe, F.W.E.; Samyn, Y. Taxonomy of the heavily exploited Indo-Pacific sandfish complex (Echinodermata: Holothuriidae). Zool. J. Linn. Soc. 2009, 155, 40–59. [Google Scholar] [CrossRef]
- Van Dyck, S.; Gerbaux, P.; Flammang, P. Elucidation of molecular diversity and body distribution of saponins in the sea cucumber Holothuria forskali (Echinodermata) by mass spectrometry. Comp. Biochem. Physiol. B 2009, 152, 124–134. [Google Scholar] [CrossRef]
- Matsuno, T.; Ishida, T. Distribution and seasonal variation of toxic principles of sea-cucumber (Holothuria leucospilota; Brandt). Cell. Mol. Life Sci. 1969, 25, 1261. [Google Scholar] [CrossRef]
- Elyakov, G.B.; Stonik, V.A.; Levina, E.V.; Slanke, V.P.; Kuznetsova, T.A.; Levin, V.S. Glycosides of marine invertebrates—I. A comparative study of the glycoside fractions of pacific sea cucumbers. Comp. Biochem. Physiol. B 1973, 44, 325–336. [Google Scholar]
- Schöpke, T.; Thiele, H.; Wray, V.; Nimtz, M.; Hiller, K. Structure elucidation of a glycoside of 2β, 3β, t23-trihydroxy-16-oxoolean-12-en-28-oic acid from bellis bernardii using mass spectrometry for the sugar sequence determination. J. Nat. Prod. 1995, 58, 152–155. [Google Scholar] [CrossRef]
- Cui, M.; Song, F.; Zhou, Y.; Liu, Z.; Liu, S. Rapid identification of saponins in plant extracts by electrospray ionization multI-stage tandem mass spectrometry and liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2000, 14, 1280–1286. [Google Scholar] [CrossRef]
- Liu, S.; Cui, M.; Liu, Z.; Song, F.; Mo, W. Structural analysis of saponins from medicinal herbs using electrospray ionization tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 133–141. [Google Scholar] [CrossRef]
- Wang, X.; Sakuma, T.; Asafu-Adjaye, E.; Shiu, G.K. Determination of ginsenosides in plant extracts from Panax ginseng and Panax quinquefolius L. by LC/MS/MS. Anal. Chem. 1999, 71, 1579–1584. [Google Scholar] [CrossRef]
- Wolfender, J.L.; Rodriguez, S.; Hostettmann, K. Liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectroscopy for the screening of plant constituents. J. Chromatogr. 1998, 794, 299–316. [Google Scholar] [CrossRef]
- Bankefors, J.; Broberg, S.; Nord, L.I.; Kenne, L. Electrospray ionization ion-trap multiple-stage mass spectrometry of Quillaja saponins. J. Mass Spectrom. 2011, 46, 658–665. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, W.; Kong, L.; Liang, M.; Li, H.; Lin, M.; Liu, R.; Zhang, C. Rapid identification of C21 steroidal saponins in Cynanchum versicolor Bunge by electrospray ionization multi-stage tandem mass spectrometry and liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 279–285. [Google Scholar] [CrossRef]
- Song, F.; Cui, M.; Liu, Z.; Yu, B.; Liu, S. Multiple-stage tandem mass spectrometry for differentiation of isomeric saponins. Rapid Commun. Mass Spectrom. 2004, 18, 2241–2248. [Google Scholar] [CrossRef]
- Van Setten, D.C.; Jan ten Hove, G.; Wiertz, E.J. H.J.; Kamerling, J.P.; van de Werken, G. Multiple-stage tandem mass spectrometry for structural characterization of saponins. Anal. Chem. 1998, 70, 4401–4409. [Google Scholar]
- Rodriguez, J.; Castro, R.; Riguera, R. Holothurinosides: New antitumour non sulphated triterpenoid glycosides from the sea cucumber holothuria forskalii. Tetrahedron 1991, 47, 4753–4762. [Google Scholar] [CrossRef]
- Kitagawa, I.; Kobayashi, M.; Kyogoku, Y. Marine natural products. IX. Structural elucidation of triterpenoidal oligoglycosides from the Bahamean sea cucumber Actinopyga agassizi Selenka. Chem. Pharm. Bull. (Tokyo) 1982, 30, 2045–2050. [Google Scholar] [CrossRef]
- Liu, B.S.; Yi, Y.H.; Li, L.; Sun, P.; Han, H.; Sun, G.Q.; Wang, X.H.; Wang, Z.L. Argusides D and E, two new cytotoxic triterpene glycosides from the sea cucumber Bohadschia argus Jaeger. Chem. Biodivers. 2008, 5, 1425–1433. [Google Scholar] [CrossRef]
- Van Dyck, S.; Flammang, P.; Meriaux, C.; Bonnel, D.; Salzet, M.; Fournier, I.; Wisztorski, M. Localization of secondary metabolites in marine invertebrates: Contribution of MALDI MSI for the study of saponins in Cuvierian tubules of H. forskali. PLoS One 2010, 5, e13923. [Google Scholar]
- Wu, J.; Yi, Y.; Zou, Z. Two new triterpene glycosides from sea cucumber Holothuria nobilis. Chin. Tradit. Herbal Drugs 2006, 37, 497. [Google Scholar]
- Han, H.; Yi, Y.H.; Li, L.; Liu, B.S.; La, M.P.; Zhang, H.W. Antifungal active triterpene glycosides from sea cucumber Holothuria scabra. Acta Pharm. Sin. 2009, 44, 620–624. [Google Scholar]
- Han, H.; Li, L.; Yi, Y.-h.; Wang, X.-h.; Pan, M.-x. Triterpene glycosides from sea cucumber Holothuria scabra with cytotoxic activity. Chin. Herb. Med. 2012, 4, 183–188. [Google Scholar]
- Kitagawa, I.; Nishino, T.; Kyogoku, Y. Structure of holothurin A a biologically active triterpene-oligoglycoside from the sea cucumber Holothuria leucospilota Brandt. Tetrahedron Lett. 1979, 20, 1419–1422. [Google Scholar] [CrossRef]
- Thanh, N.V.; Dang, N.H.; Kiem, P.V.; Cuong, N.X.; Huong, H.T.; Minh, C.V. A new triterpene glycoside from the sea cucumber Holothuria scabra collected in Vietnam. ASEAN J. Sci. Technol. Dev. 2006, 23, 253–259. [Google Scholar]
- Yuan, W.; Yi, Y.; Tang, H.; Xue, M.; Wang, Z.; Sun, G.; Zhang, W.; Liu, B.; Li, L.; Sun, P. Two new holostan-type triterpene glycosides from the sea cucumber Bohadschia marmorata JAEGER. Chem. Pharm. Bull. (Tokyo) 2008, 56, 1207–1211. [Google Scholar] [CrossRef]
- Chanley, J.D.; Ledeen, R.; Wax, J.; Nigrelli, R.F.; Sobotka, H. Holothurin. I. The isolation, properties and sugar components of holothurin A. J. Am. Chem. Soc. 1959, 81, 5180–5183. [Google Scholar] [CrossRef]
- Elyakov, G.B.; Kuznetsova, T.A.; Stonik, V.A.; Levin, V.S.; Albores, R. Glycosides of marine invertebrates. IV. A comparative study of the glycosides from Cuban sublittoral holothurians. Comp. Biochem. Physiol. B 1975, 52, 413–417. [Google Scholar]
- Yasumoto, T.; Nakamura, K.; Hashimoto, Y. A new saponin, holothurin B, isolated from sea-cucumber, Holothuria vagabunda and Holothuria lubrica. Agric. Biol. Chem. 1967, 31, 7–10. [Google Scholar] [CrossRef]
- Matsuno, T.; Iba, J. Studies on the saponins of the sea cucumber. Yakugaku Zasshi 1966, 86, 637–638. [Google Scholar]
- Silchenko, A.S.; Stonik, V.A.; Avilov, S.A.; Kalinin, V.I.; Kalinovsky, A.I.; Zaharenko, A.M.; Smirnov, A.V.; Mollo, E.; Cimino, G. Holothurins B2, B3, and B4, new triterpene glycosides from mediterranean sea cucumbers of the genus holothuria. J. Nat. Prod. 2005, 68, 564–567. [Google Scholar] [CrossRef]
- Sun, G.Q.; Li, L.; Yi, Y.H.; Yuan, W.H.; Liu, B.S.; Weng, Y.Y.; Zhang, S.L.; Sun, P.; Wang, Z.L. Two new cytotoxic nonsulfated pentasaccharide holostane (=20-hydroxylanostan-18-oic acid γ-lactone) glycosides from the sea cucumber Holothuria grisea. Helv. Chim. Acta 2008, 91, 1453–1460. [Google Scholar] [CrossRef]
- Zou, Z.; Yi, Y.; Wu, H.; Yao, X.; Du, L.; Jiuhong, W.; Liaw, C.C.; Lee, K.H. Intercedensides D−I, cytotoxic triterpene glycosides from the sea cucumber Mensamaria intercedens Lampert. J. Nat. Prod. 2005, 68, 540–546. [Google Scholar] [CrossRef]
- Sottorff, I.; Aballay, A.; Hernández, V.; Roa, L.; Muñoz, L.X.; Silva, M.; Becerra, J.; Astuya, A. Characterization of bioactive molecules isolated from sea cucumber Athyonidium chilensis. Rev. Biol. Mar. Oceanogr. 2013, 48, 23–35. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Dolmatov, I.Y.; Kalinin, V.I.; Stonik, V.A. Structure and biological action of Cladolosides B1, B2, C, C1, C2 and D, six new triterpene glycosides from the sea cucumber Cladolabes schmeltzii. Nat. Prod. Commun. 2013, 8, 1527–1534. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Kalinin, V.I. Structures and cytotoxic properties of cucumariosides H2, H3 and H4 from the sea cucumber Eupentacta fraudatrix. Nat. Prod. Res. 2012, 26, 1765–1774. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Britton, R.W.; Ziegler, M.F.; Sigel, C.W. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973, 38, 178–179. [Google Scholar] [CrossRef]
- Oleszek, W.; Marston, A. Saponins in Food, Feedstuffs and Medicinal Plants; Springer: Dordrecht, The Netherlands, 2000; Volume 45. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bahrami, Y.; Zhang, W.; Chataway, T.; Franco, C. Structural Elucidation of Novel Saponins in the Sea Cucumber Holothuria lessoni. Mar. Drugs 2014, 12, 4439-4473. https://doi.org/10.3390/md12084439
Bahrami Y, Zhang W, Chataway T, Franco C. Structural Elucidation of Novel Saponins in the Sea Cucumber Holothuria lessoni. Marine Drugs. 2014; 12(8):4439-4473. https://doi.org/10.3390/md12084439
Chicago/Turabian StyleBahrami, Yadollah, Wei Zhang, Tim Chataway, and Chris Franco. 2014. "Structural Elucidation of Novel Saponins in the Sea Cucumber Holothuria lessoni" Marine Drugs 12, no. 8: 4439-4473. https://doi.org/10.3390/md12084439