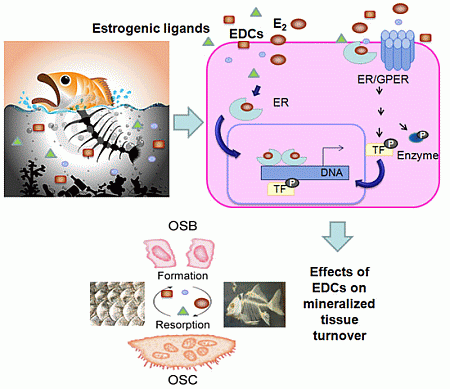
Effects of Estrogens and Estrogenic Disrupting Compounds on Fish Mineralized Tissues
Abstract
:1. Introduction

2. Mineralized Tissues and Mineral Homeostasis
3. Estrogen Actions in Mineralized Tissues
4. General Mechanisms of Estrogenic Action

5. Mechanisms of Estrogenic Action in Mineralized Tissues
| Species | Tissue | Transcript/Protein and Detection Method | ERα | ERβa | ERβb | References |
|---|---|---|---|---|---|---|
| Sparus auratus | Endochondral bone (jaw) | Transcript (qRT-PCR) | √ (low) | √ | √ | [72] |
| Sparus auratus | Dermal bone (skull) | Transcript (RT-PCR) | - | √ | √ | [46] |
| Sparus auratus | Perichondral bone (vertebral centra) | Transcript (qRT-PCR) | √ (low) | √ | √ | [72,73] |
| Sparus auratus | Chondroid bone (gill arches) | Transcript (qRT-PCR) | √ (low) | √ | √ | [73] |
| Sparus auratus | Cartilage (Intervertebral disc) | Transcript (RT-PCR) | - | √ | √ | [46] |
| S. auratus; Oreochromis mossambicus; Carassius auratus; Oncorhynchus mykiss; | Scales | Transcript (RT-PCR); Protein (IHC) | √ (low) | √ | √ | [32,40,46] |
| Sparus auratus | Skin with scales | Transcript (qRT-PCR) | √ (low) | √ | √ | [42] |
6. Estrogenic Endocrine Disruption
- (1)
- The high number of natural and anthropogenic chemical compounds with structural similarity to natural estrogens [78];
- (2)
- (3)
- (4)
| Factor | Influence | |
|---|---|---|
| Structure of the chemical: | Determines binding to a given receptor and the resulting receptor conformation (agonist or antagonist-type) | |
| Cellular context: | Diversity and functional characteristics of receptors | The expression, sub-cellular localization and functional characteristics of intracellular ERs, their variants or membrane ERs/GPERs determine the signaling pathways that are activated or repressed |
| Diversity of coregulators | The cellular context in terms of the presence and levels of co-repressors and/or co-activators greatly influences cell-specific effects on an estrogenic ligand | |
| Diversity of other transcription factors | The diversity of other transcription factors influences the possibility of indirect actions on alternative genes | |
7. Estrogenic Endocrine Disruption in Mineralized Tissues
- (1)
- The diverse fish species of ecological and commercial interest, in the wild or reared in aquaculture units, which may have different responses;
- (2)
- The endpoints that should be evaluated to assess EDC effects and elucidate the mode of action;
- (3)
- The tissue-specific responses to EDCs. For example, scales are proposed to be a preferential site for E2-induced Ca mobilization compared to bone [33,39], and they are directly exposed to the aquatic environment and for these reasons have been preferentially studied as an EDC target tissue (Table 1). However, the estrogenic EDCs impacts on the fish endoskeleton and different bone types (endochondral, dermal, chondroid) need to be studied, as they respond differently to estrogenic compounds [46,72];
- (4)
- The number of contaminants present in the aquatic environment both alone (in different concentrations) or in complex mixtures that may have an estrogenic disrupting action.
| Species | Compound | Effective Dose | Exposure Type and Period | Endpoint | Effect | Reference |
|---|---|---|---|---|---|---|
| Pimephales promelas | 17α-ethynylestradiol (EE2) | 0.1 to 100 μg/L | In vivo, from 24 hpf to 25–26 dph | Degree of skeletal development; spinal abnormalities | Modified skeletal developmental; vertebral malformations in up to 62% of fish | [86] |
| Fundulus heteroclitus | 17α-ethynylestradiol (EE2) | 1000 to 10,000 ng/L 10 and 10,000 ng/L | In vivo, first 25 or 60 day of life | Skeletal and soft tissue abnormalities | Increased % of abnormal fish; increased number of abnormalities per fish | [83] |
| Gambusia holbrooki | Sewage (two sewage treatment plants) | n.a. | n.a. | Hemal spines morphology | Modified hemal spines with one sewage source | [88] |
| Carassius auratus | Bisphenol A (BPA) | 10−6 to 10−5 M | In vitro, scale assay (6 h) | TRAP and ALP activity; transcript expression | Suppressed OSB and OSC activity; no changes in IGF-I expression | [90] |
| Carassius auratus (freshwater); Girella punctata and Pseudolabrus sieboldi (marine) | Tributyltin acetate (TBTA) | 10−9 to 10−5 M | In vitro, scale assay (6 h) | TRAP and ALP activity | Inhibits OSB activity; no effect on OSC activity | [91] |
| Carassius auratus (freshwater); Pseudolabrus sieboldi (marine) | 3- and 4-OHBaA | 10−7 to 10−5 M | In vitro, scale assay (6 and 18 h) | TRAP and ALP activity; transcript expression | Inhibited OSB and OSC activities 4-OHBaA down-regulated cathepsin K and IGF-I expression | [37] |
| Carassius auratus | Polychlorinated biphenyl (PCB 118) | 100 ng/g BW 0.0025–2.5 ppm | In vivo, intraperitoneal injection (2 days) In vitro, scale assay (6 and 18 h) | TRAP and ALP activity in scales Ca level in plasma transcript expression | Increased OSC activity; hypercalcemia; increased OSC and OBS activity; upregulated cathepsin K, TRAP and RANKL expression | [92] |
| Sparus auratus | Raloxifene | 3.33 mg/kg BW | In vivo, intraperitoneal injection (6 days) | Ca level in plasma balance; transcript expression in dermal and perichondral bone | No change in Ca levels; downregulation of genes related to bone formation and resorption in vertebra (perichondral bone) | [72] |
8. Conclusions
- (1)
- Screening of a far greater number of EDCs, including endpoints, such as the assessment of enzyme activities, gene expression, proteome changes and gene networks and cellular pathways;
- (2)
- Characterization of tissue-specific responses (e.g., bone type and bone versus scale);
- (3)
- Establishment of species-specific, season-specific and age-specific responses;
- (4)
- Determination of the impact of estrogenic disruption on fish health and survival.
Acknowledgments
Conflicts of Interest
References
- Bentley, P.J. Comparative Vertebrate Endocrinology, 3rd ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Guerreiro, P.M.; Fuentes, J.; Canario, A.V.; Power, D.M. Calcium balance in sea bream (Sparus aurata): The effect of oestradiol-17beta. J. Endocrinol. 2002, 173, 377–385. [Google Scholar] [CrossRef]
- Lange, I.G.; Hartel, A.; Meyer, H.H. Evolution of oestrogen functions in vertebrates. J. Steroid Biochem. Mol. Biol. 2002, 83, 219–226. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Zhu, P.; Li, J.; Sham, K.W.; Cheng, S.H.; Li, S.; Zhang, Y.; Cheng, C.H.; Lin, H. G-protein-coupled estrogen receptor 1 is involved in brain development during zebrafish (Danio rerio) embryogenesis. Biochem. Biophys. Res. Commun. 2013, 435, 21–27. [Google Scholar] [CrossRef] [PubMed]
- International Programme on Chemical Safety (IPCS), Global Assessment of the State-of-the-Science of Endocrine Disruptors. World Health Organization, 2002. Available online: http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/ (accessed on 1 March 2014).
- Marino, M.; Pellegrini, M.; La Rosa, P.; Acconcia, F. Susceptibility of estrogen receptor rapid responses to xenoestrogens: Physiological outcomes. Steroids 2012, 77, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Ropero, A.B.; Alonso-Magdalena, P.; Ripoll, C.; Fuentes, E.; Nadal, A. Rapid endocrine disruption: Environmental estrogen actions triggered outside the nucleus. J. Steroid Biochem. Mol. Biol. 2006, 102, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.M.; Blumberg, B. New modes of action for endocrine-disrupting chemicals. Mol. Endocrinol. 2006, 20, 475–482. [Google Scholar] [CrossRef] [PubMed]
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar]
- Iwanowicz, L.R.; Blazer, V.S. A brief overview of estrogen-associated endocrine in fishes: Evidence of effects on reproductive and immune physiology. In Aquatic Animal Health: A Continuing Dialogue between Russia and the United States, Proceedings of the 3rd Bilateral Conference between the United States and Russia, Shepherdstown, WV, USA, 12–20 July 2009; Cipriano, R.C., Bruckner, A., Shchelkunov, I.S., Eds.; Khaled bin Sultan Living Oceans Foundation: Landover, MD, USA, 2011. [Google Scholar]
- Scholz, S.; Mayer, I. Molecular biomarkers of endocrine disruption in small model fish. Mol. Cell. Endocrinol. 2008, 293, 57–70. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme (UNEP); World Health Organization (WHO). The State-of-the-Science of Endocrine Disrupting Chemicals—2012. UNEP/WHO 2013. Bergman, A., Heindel, J.J., Jobling, S., Kidd, K.A., Zoeller, R.T., Eds.; Available online: http://www.who.int/ceh/publications/endocrine/en/ (accessed on 1 March 2014).
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar]
- Kloas, W.; Urbatzka, R.; Opitz, R.; Wurtz, S.; Behrends, T.; Hermelink, B.; Hofmann, F.; Jagnytsch, O.; Kroupova, H.; Lorenz, C.; et al. Endocrine disruption in aquatic vertebrates. Ann. N. Y. Acad. Sci. 2009, 1163, 187–200. [Google Scholar] [CrossRef]
- Zhang, L.; Sedykh, A.; Tripathi, A.; Zhu, H.; Afantitis, A.; Mouchlis, V.D.; Melagraki, G.; Rusyn, I.; Tropsha, A. Identification of putative estrogen receptor-mediated endocrine disrupting chemicals using QSAR- and structure-based virtual screening approaches. Toxicol. Appl. Pharmacol. 2013, 272, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Bencic, D.C.; Breen, M.S.; Collette, T.W.; Conolly, R.B.; Denslow, N.D.; Edwards, S.W.; Ekman, D.R.; Garcia-Reyero, N.; Jensen, K.M.; et al. Endocrine disrupting chemicals in fish: Developing exposure indicators and predictive models of effects based on mechanism of action. Aquat. Toxicol. 2009, 92, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Oury, F. Biology without walls: The novel endocrinology of bone. Annu. Rev. Physiol. 2012, 74, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Kardong, K.V. Vertebrates: Comparative Anatomy, Function, Evolution, 2nd ed.; WCB McGraw-Hill: Boston, MA, USA, 1998. [Google Scholar]
- Meunier, F.J.; Huysseune, A. The concept of bone tissue in osteichthyes. Neth. J. Zool. 1992, 42, 445–458. [Google Scholar] [CrossRef]
- Guerreiro, P.M.; Fuentes, J. Control of Calcium Balance in Fish. In Fish Osmoregulation; Baldisserotto, B., Mancera Romero, J.M., Kapoor, B.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Moyle, P.B.; Cech, J.J., Jr. Fishes: An Introduction to Ichthyology; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Khosla, S. Update on estrogens and the skeleton. J. Clin. Endocrinol. Metab. 2010, 95, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Martin-Millan, M.; Almeida, M.; Ambrogini, E.; Han, L.; Zhao, H.; Weinstein, R.S.; Jilka, R.L.; O’Brien, C.A.; Manolagas, S.C. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol. Endocrinol. 2010, 24, 323–334. [Google Scholar]
- Matsumoto, Y.; Otsuka, F.; Takano-Narazaki, M.; Katsuyama, T.; Nakamura, E.; Tsukamoto, N.; Inagaki, K.; Sada, K.E.; Makino, H. Estrogen facilitates osteoblast differentiation by upregulating bone morphogenetic protein-4 signaling. Steroids 2013, 78, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Oury, F. A crosstalk between bone and gonads. Ann. N. Y. Acad. Sci. 2012, 1260, 1–7. [Google Scholar] [CrossRef]
- Bromage, N.R.; Whitehead, C.; Breton, B. Relationships between serum levels of gonadotropin, oestradiol-17β, and vitellogenin in the control of ovarian development in the rainbow trout: II. The effects of alterations in environmental photoperiod. Gen. Comp. Endocrinol. 1982, 47, 366–376. [Google Scholar] [CrossRef]
- Carragher, J.F.; Sumpter, J.P. The mobilization of calcium from calcified tissues of rainbow trout (Oncorhynchus mykiss) induced to synthesize vitellogenin. Comp. Biochem. Physiol. 1991, 99, 169–172. [Google Scholar] [CrossRef]
- Li, C.; Liu, R.; Wang, H.; Deng, R. The initial study on the relationship between vitellogenin and calcium ion in Carassius auratus curieri. J. Fish. China 1993, 7, 297–303. [Google Scholar]
- Norberg, B.; Bjornsson, B.T.; Brown, C.L.; Wichardt, U.P.; Deftos, L.J.; Haux, C. Changes in plasma vitellogenin, sex steroids, calcitonin, and thyroid hormones related to sexual maturation in female brown trout (Salmo trutta). Gen. Comp. Endocrinol. 1989, 75, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.; Sundell, K.; Bjornsson, B.T. Estradiol-17β-induced calcium uptake and resorption in juvenile rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 1994, 13, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Armour, K.J.; Lehane, D.B.; Pakdel, F.; Valotaire, Y.; Graham, R.; Russell, R.G.; Henderson, I.W. Estrogen receptor mRNA in mineralized tissues of rainbow trout: Calcium mobilization by estrogen. FEBS Lett. 1997, 411, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Mugiya, Y.; Watabe, N. Studies on fish scale formation and resorption—II. Effect of estradiol on calcium homeostasis and skeletal tissue resorption in the goldfish, Carassius auratus, and the killifish, Fundulus heteroclitus. Comp. Biochem. Physiol. Part A Physiol. 1977, 57, 197–202. [Google Scholar]
- Persson, P.; Takagi, T.; Björnsson, B.T. Tartrate resistant acid phosphatase as a marker for scale resorption in rainbow trout, Oncorhynchus mykiss: Effects of estradiol-17β treatment and refeeding. Fish Physiol. Biochem. 1995, 14, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Rotllant, J.; Redruello, B.; Guerreiro, P.M.; Fernandes, H.; Canario, A.V.; Power, D.M. Calcium mobilization from fish scales is mediated by parathyroid hormone related protein via the parathyroid hormone type 1 receptor. Regul. Pept. 2005, 132, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hattori, A. Melatonin suppresses osteoclastic and osteoblastic activities in the scales of goldfish. J. Pineal Res. 2002, 33, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hayakawa, K.; Kameda, T.; Triba, A.; Tang, N.; Tabata, M.J.; Takada, K.; Wada, S.; Omori, K.; Srivastav, A.K.; et al. Monohydroxylated polycyclic aromatic hydrocarbons inhibit both osteoclastic and osteoblastic activities in teleost scales. Life Sci. 2009, 84, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Suzuki, T.; Kurokawa, T. Suppression of osteoclastic activities by calcitonin in the scales of goldfish (freshwater teleost) and nibbler fish (seawater teleost). Peptides 2000, 21, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.; Johannsson, S.H.; Takagi, Y.; Bjornsson, B.T. Estradiol-17β and nutritional status affect calcium balance, scale and bone resorption, and bone formation in rainbow trout, Oncorhynchus mykiss. J. Comp. Physiol. Part B Biochem. Syst. Environ. Physiol. 1997, 167, 468–473. [Google Scholar]
- Yoshikubo, H.; Suzuki, N.; Takemura, K.; Hoso, M.; Yashima, S.; Iwamuro, S.; Takagi, Y.; Tabata, M.J.; Hattori, A. Osteoblastic activity and estrogenic response in the regenerating scale of goldfish, a good model of osteogenesis. Life Sci. 2005, 76, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Rawson, C.A.; Lim, R.P.; Warne, M.S.; Doyle, C.J. The effect of 17beta-estradiol on the development of modified hemal spines in early-life stage Gambusia holbrooki. Arch. Environ. Contam. Toxicol. 2006, 51, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ibarz, A.; Pinto, P.I.; Power, D.M. Proteomic approach to skin regeneration in a marine teleost: Modulation by oestradiol-17beta. Mar. Biotechnol. (NY) 2013, 15, 629–646. [Google Scholar] [CrossRef]
- Bollig, A.; Miksicek, R.J. An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Mol. Endocrinol. 2000, 14, 634–649. [Google Scholar] [PubMed]
- Gonzalez, M.; Reyes, R.; Damas, C.; Alonso, R.; Bello, A.R. Oestrogen receptor alpha and beta in female rat pituitary cells: An immunochemical study. Gen. Comp. Endocrinol. 2008, 155, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.R.; Habibi, H.R. Estrogen receptor function and regulation in fish and other vertebrates. Gen. Comp. Endocrinol. 2013, 192, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.I.; Estevao, M.D.; Redruello, B.; Socorro, S.M.; Canario, A.V.; Power, D.M. Immunohistochemical detection of estrogen receptors in fish scales. Gen. Comp. Endocrinol. 2009, 160, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Tingaud-Sequeira, A.; Andre, M.; Forgue, J.; Barthe, C.; Babin, P.J. Expression patterns of three estrogen receptor genes during zebrafish (Danio rerio) development: Evidence for high expression in neuromasts. Gene Expr. Patterns 2004, 4, 561–568. [Google Scholar]
- Wu, C.; Patino, R.; Davis, K.B.; Chang, X. Localization of estrogen receptor alpha and beta RNA in germinal and nongerminal epithelia of the channel catfish testis. Gen. Comp. Endocrinol. 2001, 124, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Bjornstrom, L.; Sjoberg, M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005, 19, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Laurentino, S.S.; Pinto, P.I.; Correia, S.; Cavaco, J.E.; Canario, A.V.; Socorro, S. Structural variants of sex steroid hormone receptors in the testis: From molecular biology to physiological roles. OA Biotechnol. 2012, 1, 4. [Google Scholar] [CrossRef]
- Watson, C.S.; Alyea, R.A.; Jeng, Y.J.; Kochukov, M.Y. Nongenomic actions of low concentration estrogens and xenoestrogens on multiple tissues. Mol. Cell. Endocrinol. 2007, 274, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Soltysik, K.; Czekaj, P. Membrane estrogen receptors—Is it an alternative way of estrogen action? J. Physiol. Pharmacol. 2013, 64, 129–142. [Google Scholar]
- Thomas, P. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen. Comp. Endocrinol. 2012, 175, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Alyea, R.; Pang, Y.; Peyton, C.; Dong, J.; Berg, A.H. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids 2010, 75, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Nagler, J.J.; Davis, T.L.; Modi, N.; Vijayan, M.M.; Schultz, I. Intracellular, not membrane, estrogen receptors control vitellogenin synthesis in the rainbow trout. Gen. Comp. Endocrinol. 2010, 167, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Bemanian, V.; Male, R.; Goksoyr, A. The aryl hydrocarbon receptor-mediated disruption of vitellogenin synthesis in the fish liver: Cross-talk between AHR- and ERalpha-signalling pathways. Comp. Hepatol. 2004, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melamed, P.; Koh, M.; Preklathan, P.; Bei, L.; Hew, C. Multiple mechanisms for Pitx-1 transactivation of a luteinizing hormone beta subunit gene. J. Biol. Chem. 2002, 277, 26200–26207. [Google Scholar] [CrossRef] [PubMed]
- Centrella, M.; McCarthy, T.L. Estrogen receptor dependent gene expression by osteoblasts—direct, indirect, circumspect, and speculative effects. Steroids 2012, 77, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Steroids and osteoporosis: The quest for mechanisms. J. Clin. Investig. 2013, 123, 1919–1921. [Google Scholar] [CrossRef] [PubMed]
- Zallone, A. Direct and indirect estrogen actions on osteoblasts and osteoclasts. Ann. N. Y. Acad. Sci. 2006, 1068, 173–179. [Google Scholar] [CrossRef]
- Marie, P.J. Transcription factors controlling osteoblastogenesis. Arch. Biochem. Biophys. 2008, 473, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F.; Colvard, D.S.; Berg, N.J.; Graham, M.L.; Mann, K.G.; Spelsberg, T.C.; Riggs, B.L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science 1988, 241, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Komm, B.S.; Terpening, C.M.; Benz, D.J.; Graeme, K.A.; Gallegos, A.; Korc, M.; Greene, G.L.; O’Malley, B.W.; Haussler, M.R. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science 1988, 241, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Spelsberg, T.C.; Subramaniam, M.; Riggs, B.L.; Khosla, S. The actions and interactions of sex steroids and growth factors/cytokines on the skeleton. Mol. Endocrinol. 1999, 13, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Pottratz, S.T.; Bellido, T.; Mocharla, H.; Crabb, D.; Manolagas, S.C. 17 beta-estradiol inhibits expression of human interleukin-6 promoter-reporter constructs by a receptor-dependent mechanism. J. Clin. Invest. 1994, 93, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Prefontaine, K.E.; Ray, P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J. Biol. Chem. 1994, 269, 12940–12946. [Google Scholar] [PubMed]
- Banerjee, S.; Chambliss, K.L.; Mineo, C.; Shaul, P.W. Recent insights into non-nuclear actions of estrogen receptor alpha. Steroids 2014, 81, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Bartell, S.M.; Han, L.; Kim, H.N.; Kim, S.H.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S.; Chambliss, K.L.; Shaul, P.W.; Roberson, P.K.; Weinstein, R.S.; et al. Non-nuclear-initiated actions of the estrogen receptor protect cortical bone mass. Mol. Endocrinol. 2013, 27, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S.; Bellido, T.; Plotkin, L.I.; O’Brien, C.A.; Bodenner, D.L.; Han, L.; Han, K.; DiGregorio, G.B.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S.; et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell 2001, 104, 719–730. [Google Scholar] [PubMed]
- Almeida, M.; Iyer, S.; Martin-Millan, M.; Bartell, S.M.; Han, L.; Ambrogini, E.; Onal, M.; Xiong, J.; Weinstein, R.S.; Jilka, R.L.; et al. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J. Clin. Invest. 2013, 123, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Agas, D.; Sabbieti, M.G.; Marchetti, L. Endocrine disruptors and bone metabolism. Arch. Toxicol. 2013, 87, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.A.; Pinto, P.I.; Guerreiro, P.M.; Power, D.M. Divergent responsiveness of the dentary and vertebral bone to a selective estrogen-receptor modulator (SERM) in the teleost Sparus auratus. Gen. Comp. Endocrinol. 2012, 179, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.A.; Thorne, M.A.; Stueber, K.; Darias, M.; Reinhardt, R.; Clark, M.S.; Gisbert, E.; Power, D.M. Comparative analysis of a teleost skeleton transcriptome provides insight into its regulation. Gen. Comp. Endocrinol. 2013, 191, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.I.S.; Estêvão, M.D.; Andrade, A.; Santos, S.; Power, D.M.; Centre of Marine Sciences, University of Algarve, Faro, Portugal. Unpublished work. 2014.
- Canario, A.V.; Rotllant, J.; Fuentes, J.; Guerreiro, P.M.; Rita Teodosio, H.; Power, D.M.; Clark, M.S. Novel bioactive parathyroid hormone and related peptides in teleost fish. FEBS Lett. 2006, 580, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Danks, J.A.; Maruyama, Y.; Ikegame, M.; Sasayama, Y.; Hattori, A.; Nakamura, M.; Tabata, M.J.; Yamamoto, T.; Furuya, R.; et al. Parathyroid hormone 1 (1–34) acts on the scales and involves calcium metabolism in goldfish. Bone 2011, 48, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Guerreiro, P.M.; Modesto, T.; Rotllant, J.; Canario, A.V.; Power, D.M. A PTH/PTHrP receptor antagonist blocks the hypercalcemic response to estradiol-17beta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R956–R960. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S. State of the science of endocrine disruptors. Environ. Health Perspect. 2013, 121, A107. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.M.; Fang, H.; Branham, W.S.; Hass, B.S.; Dial, S.L.; Moland, C.L.; Tong, W.; Shi, L.; Perkins, R.; Sheehan, D.M. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: Structural diversity of ligands. Toxicol. Sci. 2000, 54, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H.; Ayers, S.; Gescher, A.J.; Glatt, H.R.; Meinl, W.; Jarratt, P.; Kirk, C.J.; Pettitt, T.; Rea, D.; Harris, R.M. Phytoestrogens and xenoestrogens: The contribution of diet and environment to endocrine disruption. J. Steroid Biochem. Mol. Biol. 2008, 108, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.M.; Milnes, M.R.; Lundberg, R.; Bermudez, D.; Orberg, J.A.; Guillette, L.J., Jr. Abnormal bone composition in female juvenile American alligators from a pesticide-polluted lake (Lake Apopka, Florida). Environ. Health Perspect. 2004, 112, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, R.; Jenssen, B.M.; Leiva-Presa, A.; Ronn, M.; Hernhag, C.; Wejheden, C.; Larsson, S.; Orberg, J.; Lind, P.M. Effects of short-term exposure to the DDT metabolite p,p'-DDE on bone tissue in male common frog (Rana temporaria). J. Toxicol. Environ. Health A 2007, 70, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.; Courtenay, S.C.; MacLatchy, D.L.; Berube, C.H.; Parrott, J.L.; van der Kraak, G.J. Utility of morphological abnormalities during early-life development of the estuarine mummichog, Fundulus heteroclitus, as an indicator of estrogenic and antiestrogenic endocrine disruption. Environ. Toxicol. Chem. 2004, 23, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Olufsen, M.; Arukwe, A. Developmental effects related to angiogenesis and osteogenic differentiation in salmon larvae continuously exposed to dioxin-like 3,3′,4,4′-tetrachlorobiphenyl (congener 77). Aquat. Toxicol. 2011, 105, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, H.; Dong, W.; Ogawa, S.; Tsukiyama, S.; Okuhara, Y.; Niiyama, M.; Ueno, N.; Peterson, R.E.; Hiraga, T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: Altered regional blood flow and impaired lower jaw development. Toxicol. Sci. 2002, 65, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Warner, K.E.; Jenkins, J.J. Effects of 17alpha-ethinylestradiol and bisphenol A on vertebral development in the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2007, 26, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Renner, P.; Belanger, S.E.; Busquet, F.; Davi, R.; Demeneix, B.A.; Denny, J.S.; Leonard, M.; McMaster, M.E.; Villeneuve, D.L.; et al. Alternatives to in vivo tests to detect endocrine disrupting chemicals (EDCs) in fish and amphibians-screening for estrogen, androgen and thyroid hormone disruption. Crit. Rev. Toxicol. 2013, 43, 45–72. [Google Scholar] [CrossRef] [PubMed]
- Rawson, C.A.; Lim, R.P.; Warne, M.S. Skeletal morphology and maturation of male Gambusia holbrooki exposed to sewage treatment plant effluent. Ecotoxicol. Environ. Saf. 2008, 70, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Witten, P.E.; Bendahmane, M.; Abou-Haila, A. Enzyme histochemical characteristics of osteoblasts and mononucleated osteoclasts in a teleost fish with acellular bone (Oreochromis niloticus, Cichlidae). Cell Tissue Res. 1997, 287, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hattori, A. Bisphenol A suppresses osteoclastic and osteoblastic activities in the cultured scales of goldfish. Life Sci. 2003, 73, 2237–2247. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Tabata, M.J.; Kambegawa, A.; Srivastav, A.K.; Shimada, A.; Takeda, H.; Kobayashi, M.; Wada, S.; Katsumata, T.; Hattori, A. Tributyltin inhibits osteoblastic activity and disrupts calcium metabolism through an increase in plasma calcium and calcitonin levels in teleosts. Life Sci. 2006, 78, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Yachiguchi, K.; Matsumoto, N.; Haga, Y.; Suzuki, M.; Matsumura, C.; Tsurukawa, M.; Okuno, T.; Nakano, T.; Kawabe, K.; Kitamura, K.I.; et al. Polychlorinated biphenyl (118) activates osteoclasts and induces bone resorption in goldfish. Environ. Sci. Pollut. Res. Int. 2014, 21, 6365–6372. [Google Scholar]
- Matthews, J.; Zacharewski, T. Differential binding affinities of PCBs, HO-PCBs, and aroclors with recombinant human, rainbow trout (Onchorhynkiss mykiss), and green anole (Anolis carolinensis) estrogen receptors, using a semi-high throughput competitive binding assay. Toxicol. Sci. 2000, 53, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Passos, A.L.; Pinto, P.I.; Power, D.M.; Canario, A.V. A yeast assay based on the gilthead sea bream (teleost fish) estrogen receptor beta for monitoring estrogen mimics. Ecotoxicol. Environ. Saf. 2009, 72, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Shyu, C.; Cavileer, T.D.; Nagler, J.J.; Ytreberg, F.M. Computational estimation of rainbow trout estrogen receptor binding affinities for environmental estrogens. Toxicol. Appl. Pharmacol. 2011, 250, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.H.; Ankley, G.T.; Segner, H.; Tyler, C.R. Screening and testing for endocrine disruption in fish-biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environ. Health Perspect. 2006, 114 (Suppl. S1), 106–114. [Google Scholar]
- Organisation for Economic Co-Operation and Development. Information on OECD Work Related to Endocrine Disrupters. 2012. Available online: http://www.oecd.org/chemicalsafety/testing/50067203.pdf (accessed on 1 March 2014).
- U.S. Environmental Protection Agency (EPA). Endocrine Disruptor Screening Program (EDSP): Universe of Chemicals and General Validation Principles—White Paper. 2012. Available online: http://www.epa.gov/endo/pubs/edsp-chemical-universe-and-general-validations-white-paper-11-12.pdf (accessed on 1 March 2014). [Google Scholar]
- Ankley, G.T.; Gray, L.E. Cross-species conservation of endocrine pathways: A critical analysis of tier 1 fish and rat screening assays with 12 model chemicals. Environ. Toxicol. Chem. 2013, 32, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Benninghoff, A.D.; Bisson, W.H.; Koch, D.C.; Ehresman, D.J.; Kolluri, S.K.; Williams, D.E. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol. Sci. 2011, 120, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.B.; Thomas, P. The unusual binding properties of the third distinct teleost estrogen receptor subtype ERb are accompanied by highly conserved amino acid changes in the ligand binding domain. Endocrinology 2004, 145, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Latonnelle, K.; Fostier, A.; Le Menn, F.; Bennetau-Pelissero, C. Binding affinities of hepatic nuclear estrogen receptors for phytoestrogens in rainbow trout (Oncorhynchus mykiss) and Siberian sturgeon (Acipenser baeri). Gen. Comp. Endocrinol. 2002, 129, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Tollefsen, K.E.; Mathisen, R.; Stenersen, J. Estrogen mimics bind with similar affinity and specificity to the hepatic estrogen receptor in Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2002, 126, 14–22. [Google Scholar] [CrossRef] [PubMed]
- European Chemical Agency (ECHA). Guidance on Information Requirements and Chemical Safety Assessment Chapter R.7b: Endpoint Specific Guidance. 2008. Available online: http://echa.europe.eu (accessed on 1 March 2014).
- Ankley, G.T.; Johnson, R.D. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. ILAR J. 2004, 45, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.H.; Pickford, D.B. Ecological risk assessment and testing for endocrine disruption in the aquatic environment. Toxicology 2002, 181–182, 383–387. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pinto, P.I.S.; Estêvão, M.D.; Power, D.M. Effects of Estrogens and Estrogenic Disrupting Compounds on Fish Mineralized Tissues. Mar. Drugs 2014, 12, 4474-4494. https://doi.org/10.3390/md12084474
Pinto PIS, Estêvão MD, Power DM. Effects of Estrogens and Estrogenic Disrupting Compounds on Fish Mineralized Tissues. Marine Drugs. 2014; 12(8):4474-4494. https://doi.org/10.3390/md12084474
Chicago/Turabian StylePinto, Patricia I. S., Maria D. Estêvão, and Deborah M. Power. 2014. "Effects of Estrogens and Estrogenic Disrupting Compounds on Fish Mineralized Tissues" Marine Drugs 12, no. 8: 4474-4494. https://doi.org/10.3390/md12084474