Augmenting Anti-Cancer Natural Products with a Small Molecule Adjuvant
Abstract
:1. Introduction
2. Results
2.1. Test Compounds

2.2. Neuro-2a Cells Are Sensitive to Euglenophycin and Glycolipid 652 Induced Toxicity


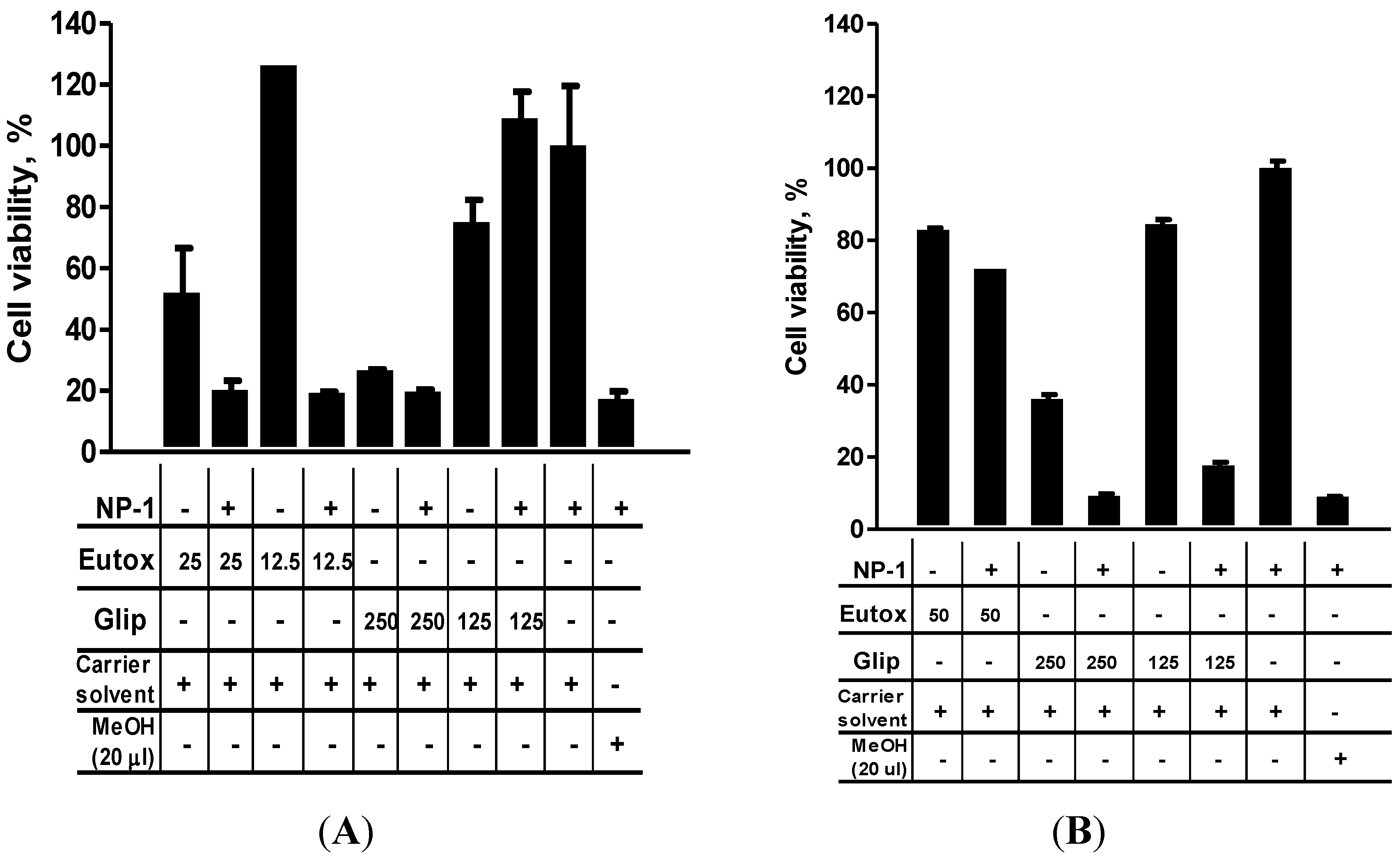
2.3. NP-1 Enhances Euglenophycin and Glycolipid 652 Induced Cytotoxicity
3. Experimental Section
3.1. Materials
3.2. Test Compound Analyses
3.3. Cell Lines and Cell Culture
3.4. Cytotoxicity Assay
3.5. Cytotoxicity Enhancement by NP-1
4. Discussion and Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
NOAA Disclaimer
References
- Zimba, P.V.; Moeller, P.D.; Beauchesne, K.; Lane, H.E.; Triemer, R.E. Identification of euglenophycin—A toxin found in certain euglenoids. Toxicon 2010, 55, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Fujii, K.; Shimada, T.; Suzuki, M. Two cyclic peptides, anabaenopeptins, a third group of bioactive compounds from the cyanobacterium Anabaena flos-aquae. Tetrahedron Lett. 1995, 36, 1511–1514. [Google Scholar] [CrossRef]
- Murakami, M.; Shin, H.J.; Matsuda, H.; Ishida, K.; Yamaguchi, K. A cyclic peptide, anabaenopeptin B, from the cyanobacterium Oscillatoria agardhii. Phytochemistry 1997, 44, 449–452. [Google Scholar] [CrossRef]
- Wahome, P.G.; Pedone, A.C.; Beauchesne, K.R.; Bernan, V.S.; Moeller, P.D.R.; Carter, G.T. Drug discovery from aquatic microbial consortia. Unpublished work. 2014. [Google Scholar]
- Zimba, P.V.; Rowan, M.; Triemer, R. Identification of euglenoid algae that produce ichthyotoxin(s). J. Fish Dis. 2004, 27, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.A.; Whitehead, D.C.; Mullikin, T.; Melander, C. Synthesis and bacterial biofilm inhibition studies of ethyl N-(2-phenethyl) carbamate derivatives. Org. Biomol. Chem. 2010, 8, 3857–3859. [Google Scholar] [CrossRef] [PubMed]
- Landier, W.; Bhatia, S. Cancer Survivorship: A Pediatric Perspective. Oncologist 2008, 13, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Kitamura, H.; Yamagushi, K.; Fukuzawa, S.; Kamijima, C.; Yazawa, K.; Kuramoto, M.; Wang, G-Y-S.; Fujitani, Y.; Uemura, D. Development of chemical substances regulating biofilm formation. Bull. Chem. Soc. Jpn. 1997, 70, 3061–3069. [Google Scholar] [CrossRef]
- Schock, T.B.; Huncik, K.; Beauchesne, K.R.; Villareal, T.A.; Moeller, D.R. Identification of Trichotoxin, a novel chlorinated compound associated with the bloom forming cyanobacterium Trichodesmium thiebautii. Environ. Sci. Technol. 2011, 45, 7503–7509. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.P.; Diss, J.K.; Chioni, A.M.; Mycielska, M.E.; Pan, H.; Yamaci, R.F.; Pani, F.; Siwy, Z.; Krasowska, M.; Grzywna, Z.; et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin. Cancer Res. 2005, 11, 5381–5389. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.-Y.; Laezza, F.; Gerber, B.R.; Xiao, M.; Yamada, K.A.; Hartmann, H.; Craig, A.M.; Nerbonne, J.M.; Ornitz, D.M. Fibroblast growth factor 14 is an intracellular modulator of voltage-gated sodium channels. J. Physiol. 2005, 569.1, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Ramsdell, J.S. Voltage-dependent calcium channels regulate GH4 pituitary cell proliferation at two stages of the cell cycle. J. Cell Physiol. 1991, 146, 197–206. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahome, P.G.; Beauchesne, K.R.; Pedone, A.C.; Cavanagh, J.; Melander, C.; Zimba, P.; Moeller, P.D.R. Augmenting Anti-Cancer Natural Products with a Small Molecule Adjuvant. Mar. Drugs 2015, 13, 65-75. https://doi.org/10.3390/md13010065
Wahome PG, Beauchesne KR, Pedone AC, Cavanagh J, Melander C, Zimba P, Moeller PDR. Augmenting Anti-Cancer Natural Products with a Small Molecule Adjuvant. Marine Drugs. 2015; 13(1):65-75. https://doi.org/10.3390/md13010065
Chicago/Turabian StyleWahome, Paul G., Kevin R. Beauchesne, Anna C. Pedone, John Cavanagh, Christian Melander, Paul Zimba, and Peter D. R. Moeller. 2015. "Augmenting Anti-Cancer Natural Products with a Small Molecule Adjuvant" Marine Drugs 13, no. 1: 65-75. https://doi.org/10.3390/md13010065




