Antidiabetic Activity of Differently Regioselective Chitosan Sulfates in Alloxan-Induced Diabetic Rats
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Parameter of Differently Regioselective Sulfate Chitosans
| Species | Molecular Weight (×104) | Sulfur Content (%) | Color of Resultant | Solubility |
|---|---|---|---|---|
| H2,3,6-S | 12.4 | 14.7 | Pale yellow | Easily soluble |
| 3,6-S | 11.7 | 12.1 | White | Easily soluble |
| 3-S | 12.1 | 5.2 | Yellow | Easily soluble |
| 6-S | 13.5 | 7.6 | White | Soluble |
| L2,3,6-S | 0.9 | 14.5 | Pale yellow | Easily soluble |
| CTS | 76 | 0 | Pale yellow | Not soluble |
2.2. Structural Characterization of All Chitosan Sulfates






2.3. The Effects of Differently Regioselective Sulfate Chitosans on Body Weight
2.4. Determination of Antidiabetic Activity of Differently Regioselective Chitosan Sulfates in Vivo
| Group | Treatment | 0th Day | 6th Day | 12th Day | 18th Day | 24th Day | 30th Day |
|---|---|---|---|---|---|---|---|
| 1 | Normal control | 183.9 ± 12.1 (10) | 197.1 ± 13.6 (10) | 219.0 ± 19.1 (10) | 233.3 ± 23.0 (10) | 237.9 ± 23.9 (10) | 261.3 ± 40.3 (10) |
| 2 | Diabetic control (DC) | 171.8 ± 25.8 (13) | 178.3 ± 34.4 (12) | 184.1 ± 39.8 (11) ∆ | 191.4 ± 37.1 (11) ∆∆ | 203.2 ± 47.9 (10) | 201.8 ± 49.4 (10) ∆∆ |
| 3 | DC + phenformin hydrochloride (100 mg/kg) | 175.4 ± 31.4 (12) | 181.1 ± 33.2 (10) | 189.6 ± 35.0 (9) | 199.0 ± 34.5 (9) | 199.7 ± 41.8 (9) | 208.1 ± 45.2 (8) |
| 4 | DC + H2,3,6-S (400 mg/kg) | 174.0 ± 14.1 (9) | 191.6 ± 11.0 (7) | 178.9 ± 22.9 (7) | 200.2 ± 21.4 (6) | 209.8 ± 22.3 (6) | 213.2 ± 20.9 (6) |
| 5 | DC + H2,3,6-S (150 mg/kg) | 183.3 ± 25.7 (8) | 200.4 ± 28.8 (7) | 186.1 ± 23.9 (7) | 207.4 ± 30.3 (7) | 200.5 ± 29.5 (6) | 207.7 ± 36.6 (6) |
| 6 | DC + H2,3,6-S (50 mg/kg) | 185.9 ± 10.5 (8) | 201.3 ± 15.1 (7) | 198.4 ± 23.4 (7) | 218.7 ± 30.6 (7) | 224.4 ± 42.9 (7) | 223.3 ± 44.5 (7) |
| 7 | DC + L2,3,6-S (400 mg/kg) | 180.3 ± 14.6 (9) | 198.8 ± 8.1 (8) | 201.8 ± 11.1 (8) | 207.5 ± 31.7 (8) | 232.3 ± 27.5 (6) | 234.3 ± 41.1 (6) |
| 8 | DC + L2,3,6-S (150 mg/kg) | 170.8 ± 11.3 (8) | 188.9 ± 18.2 (7) | 187.9 ± 25.3 (7) | 199.0 ± 32.5 (7) | 213.1 ± 46.8 (7) | 205.9 ± 48.5 (7) |
| 9 | DC + L2,3,6-S (50 mg/kg) | 178.1 ± 23.7 (9) | 200.9 ± 26.0 (7) | 204.9 ± 28.3 (7) | 217.1 ± 37.4 (7) | 228.4 ± 49.1 (7) | 228.1 ± 38.8 (7) |
| 10 | DC + 6-S (400 mg/kg) | 173.4 ± 18.1 (9) | 189.5 ± 23.7 (8) | 202.4 ± 24.1 (7) | 213.0 ± 31.7 (7) | 199.0 ± 21.7 (6) | 201.6 ± 20.5 (5) |
| 11 | DC + 6-S (150 mg/kg) | 183.3 ± 13.9 (9) | 195.3 ± 18.4 (8) | 206.5 ± 23.1 (8) | 222.5 ± 36.9 (8) | 240.1 ± 43.7 (8) | 238.4 ± 41.0 (8) |
| 12 | DC + 6-S (50 mg/kg) | 179.6 ± 23.1 (8) | 189.4 ± 33.9 (8) | 196.5 ± 42.4 (8) | 201.9 ± 50.1 (8) | 222.9 ± 63.6 (8) | 223.4 ± 72.9 (8) |
| 13 | DC + 3,6-S (400 mg/kg) | 170.2 ± 20.1 (9) | ± | ± | ± | ± | ± |
| 14 | DC + 3,6-S (150 mg/kg) | 168.8 ± 6.11 (9) | ± | ± | ± | ± | ± |
| 15 | DC + 3,6-S (50 mg/kg) | 167.7 ± 15.2 (7) | 175.9 ± 16.3 (7) | 188.0 ± 29.4 (7) | 193.1 ± 34.8 (7) | 196.6 ± 46.9 (7) | 211.7 ± 47.3 (6) |
| 16 | DC + 3-S (400 mg/kg) | 173.8 ± 25.0 (9) | 195.9 ± 32.1 (8) | 200.5 ± 35.1 (8) | 215.5 ± 47.4 (8) | 221.0 ± 63.5 (8) | 221.9 ± 65.8 (8) |
| 17 | DC + 3-S (150 mg/kg) | 176.0 ± 28.5 (9) | 192.5 ± 41.0 (8) | 208.4 ± 38.6 (7) | 232.7 ± 42.7 (6) | 243.5 ± 48.8 (6) | 244.2 ± 59.9 (6) |
| 18 | DC + 3-S (50 mg/kg) | 189.9 ± 27.1 (8) | 207.6 ± 38.3 (7) | 221.2 ± 46.4 (6) | 230.8 ± 39.2 (6) | 245.8 ± 50.8 (6) | 247.2 ± 51.9 (6) |
| 19 | DC + CTS (400 mg/kg) | 183.0 ± 21.2 (9) | 200.6 ± 31.7 (7) | 207.5 ± 44.6 (6) | 214.2 ± 50.4 (5) | 221.0 ± 65.9 (5) | 236.5 ± 62.7 (4) |
| 20 | DC + CTS (150 mg/kg) | 174.7 ± 16.4 (9) | 188.1 ± 22.2 (8) | 201.0 ± 31.5 (7) | 215.1 ± 32.5 (7) | 224.6 ± 44.7 (7) | 219.0 ± 46.9 (7) |
| 21 | DC + CTS (50 mg/kg) | 184.1 ± 23.6 (9) | 197.4 ± 31.1 (7) | 194.7 ± 37.6 (7) | 198.1 ± 42.9 (7) | 203.8 ± 29.7 (5) | 212.5 ± 43.2 (4) |
| Group | Treatment | 0th Day | 6th Day | 12th Day | 18th Day | 24th Day | 30th Day |
|---|---|---|---|---|---|---|---|
| 1 | Normal control | 4.94 ± 0.64 (10) | 4.96 ± 0.39 (10) | 4.97 ± 0.37 (10) | 5.84 ± 0.89 (10) | 5.74 ± 1.13 (10) | 5.70 ± 1.06 (10) |
| 2 | Diabetic control (DC) | 23.02 ± 6.77 (13) ∆∆∆ | 24.22 ± 7.97 (12) ∆∆∆ | 26.59 ± 6.77 (11) ∆∆∆ | 27.01 ± 7.49 (11) ∆∆∆ | 24.06 ± 4.37 (10) ∆∆∆ | 21.83 ± 6.66(10) ∆∆∆ |
| 3 | DC + phenformin hydrochloride (100 mg/kg) | 24.08 ± 5.87 (12) | 20.82 ± 7.69 (10) | 17.16 ± 7.27 (9) ** | 14.73 ± 6.23 (9) *** | 13.48 ± 4.45 (9) *** | 14.84 ± 6.09 (8) * |
| 4 | DC + H2,3,6-S (400 mg/kg) | 22.60 ± 6.82 (9) | 22.84 ± 6.23 (7) | 19.74 ± 7.13 (7) | 23.88 ± 7.55 (6) | 22.12 ± 7.79 (6) | 18.12 ± 8.29 (6) |
| 5 | DC + H2,3,6-S (150 mg/kg) | 23.39 ± 6.96 (8) | 24.89 ± 6.11 (7) | 23.54 ± 7.03 (7) | 27.34 ± 3.79 (7) | 21.02 ± 5.22 (6) | 22.03 ± 6.78 (6) |
| 6 | DC + H2,3,6-S (50 mg/kg) | 22.49 ± 6.06 (8) | 19.96 ± 7.65 (7) | 20.64 ± 8.68 (7) | 18.39 ± 10.86 (7) | 16.16 ± 10.16 (7) | 17.23 ± 9.39 (7) |
| 7 | DC + L2,3,6-S (400 mg/kg) | 23.23 ± 6.57 (9) | 21.41 ± 2.29 (8) | 20.28 ± 2.88 (8) * | 17.68 ± 6.93 (8) * | 15.85 ± 10.59 (6) | 15.68 ± 8.39 (6) |
| 8 | DC + L2,3,6-S (150 mg/kg) | 22.76 ± 6.42 (8) | 20.99 ± 7.24 (7) | 21.14 ± 6.11 (7) | 22.40 ± 6.15 (7) | 22.56 ± 5.14 (7) | 23.80 ± 6.49 (7) |
| 9 | DC + L2,3,6-S (50 mg/kg) | 24.11 ± 7.58 (9) | 23.76 ± 3.17 (7) | 20.56 ± 6.73 (7) | 19.50 ± 8.274 (7) | 20.26 ± 8.91 (7) | 20.73 ± 9.69 (7) |
| 10 | DC + 6-S (400 mg/kg) | 22.26 ± 5.89 (9) | 27.11 ± 7.62 (8) | 21.57 ± 8.26 (7) | 22.46 ± 10.55 (7) | 17.52 ± 9.25 (6) | 23.50 ± 10.82 (5) |
| 11 | DC + 6-S (150 mg/kg) | 22.06 ± 6.88 (9) | 20.33 ± 7.93 (8) | 20.40 ± 7.06 (8) | 18.43 ± 9.35 (8) | 20.40 ± 9.53 (8) | 16.51 ± 9.60 (8) |
| 12 | DC + 6-S (50 mg/kg) | 22.98 ± 7.23 (8) | 26.98 ± 4.90 (8) | 19.99 ± 8.28 (8) | 19.53 ± 10.44 (8) | 19.64 ± 11.57 (8) | 16.58 ± 9.22 (8) |
| 13 | DC + 3,6-S (400 mg/kg) | 22.59 ± 5.98 (9) | ± | ± | ± | ± | ± |
| 14 | DC + 3,6-S (150 mg/kg) | 24.19 ± 7.66 (9) | ± | ± | ± | ± | ± |
| 15 | DC + 3,6-S (50 mg/kg) | 26.14 ± 6.50 (7) | 22.77 ± 8.32 (7) | 20.41 ± 6.25 (7) | 19.89 ± 5.27 (7) * | 19.73 ± 8.97 (7) | 22.08 ± 8.89 (6) |
| 16 | DC + 3-S (400 mg/kg) | 24.09 ± 7.55 (9) | 21.91 ± 9.62 (8) | 19.21 ± 7.75 (8) * | 20.31 ± 8.96 (8) * | 19.50 ± 8.14 (8) * | 22.08 ± 8.89 (8) |
| 17 | DC + 3-S (150 mg/kg) | 23.79 ± 7.77 (9) | 22.90 ± 5.10 (8) | 18.63 ± 5.22 (7) ** | 18.22 ± 3.41 (6) ** | 17.55 ± 3.29 (6) ** | 16.13 ± 4.36 (6) * |
| 18 | DC + 3-S (50 mg/kg) | 22.88 ± 7.85 (8) | 22.84 ± 6.15 (7) | 17.65 ± 4.20 (6) ** | 18.45 ± 5.14 (6) * | 22.43 ± 5.86 (6) | 18.70 ± 6.32 (6) |
| 19 | DC + CTS (400 mg/kg) | 23.94 ± 8.06 (9) | 21.37 ± 5.44 (7) | 19.75 ± 5.48 (6) * | 21.92 ± 7.72 (5) | 21.74 ± 9.90 (5) | 23.20 ± 8.00 (4) |
| 20 | DC + CTS (150 mg/kg) | 22.88 ± 7.41 (9) | 21.98 ± 7.52 (8) | 19.74 ± 6.87 (7) | 21.13 ± 9.53 (7) | 18.49 ± 8.51 (7) | 18.86 ± 9.72 (7) |
| 21 | DC + CTS (50 mg/kg) | 24.21 ± 8.24 (9) | 23.83 ± 7.90 (7) | 21.77 ± 7.91 (7) | 24.00 ± 8.78 (7) | 22.68 ± 9.09 (5) | 23.83 ± 4.35 (4) |
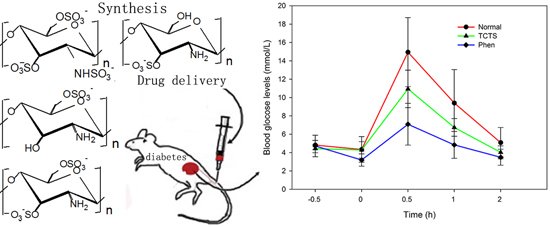
2.5. Effect of 3-S on the Sugar Tolerance of Normal Rats
| Group | Dose (mg/kg) | n | Prior to Treatment | After Treatment | |||
|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 2 h | ||||
| Normal control | 10 | 4.82 ± 0.50 | 4.34 ± 1.39 | 14.95 ± 3.76 | 9.41 ± 3.63 | 5.09 ± 1.64 | |
| 3-S | 300 | 10 | 4.43 ± 0.44 | 4.29 ± 0.90 | 10.94 ± 2.04 ** | 6.74 ± 0.97 * | 4.03 ± 0.70 * |
| Phenformin hydrochloride | 200 | 10 | 4.72 ± 1.17 | 3.19 ± 0.67 * | 7.09 ± 2.28 *** | 4.84 ± 1.46 ** | 3.49 ± 0.87 * |

2.6. The Effect of 3-S on Fasting Blood Glucose and Insulin Levels
| Group | Treatment | n | Blood Glucose Level (mmol/L) | Fasting Serum Insulin Levels µIU/mL | |
|---|---|---|---|---|---|
| Before Treatment | After Treatment | ||||
| 1 | Normal control | 10 | 5.17 ± 1.05 | 5.00 ± 0.81 | 6.71 ± 1.70 |
| 2 | Diabetic control (DC) | 10 | 32.10 ± 1.76 ∆∆∆ | 26.18 ± 5.68 ∆∆∆ | 3.54 ± 1.93 ∆∆ |
| 3 | DC + 3-S (800 mg/kg) | 10 | 30.25 ± 5.30 | 19.76 ± 9.20 * | 5.44 ± 1.65 * |
| 4 | DC + 3-S (300 mg/kg) | 10 | 29.96 ± 4.94 | 17.86 ± 7.93 ** | 5.12 ± 1.50 * |
| 5 | DC + 3-S (100 mg/kg) | 10 | 31.78 ± 3.07 | 20.05 ± 7.28 | 4.00 ± 1.65 |
| 6 | DC + Glibenclamide (25 mg/kg) | 10 | 31.92 ± 2.63 | 26.45 ± 7.00 | 4.94 ± 1.85 |
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Experiment
4.2.1. Preparation of Sulfating Reagent
4.2.2. The Preparation of Sulfated Chitosan of C2,3,6 Sulfation (H2,3,6-S)
4.2.3. The Preparation of Sulfated Chitosan of C2,3,6 Sulfation (L2,3,6-S)
4.2.4. The Preparation of Sulfated Chitosan of C3,6 Sulfation (3,6-S)
4.2.5. The Preparation of Sulfated Chitosan of C3 Sulfation (3-S)
4.2.6. The Preparation of Sulfated Chitosan of C6 Sulfation (6-S)
4.2.7. Analytical Methods
4.3. Animals
4.4. Studies on Alloxan-Induced Diabetic Rats
4.4.1. Induction of Diabetes Mellitus
4.4.2. Determination of the Hypoglycemic Effect on Diabetic Rats
4.4.3. Glucose Tolerance Test
4.4.4. Determination of the Plasma Insulin Concentration
4.5. Statistical Analysis
5. Conclusions
- Differently regioselective chitosan sulfates exhibited hypoglycemic activities.
- Hypoglycemic activity of low molecular weight sulfate chitosan was obviously higher.
- 3-S exhibited significantly hypoglycemic activities in alloxan-induced diabetic rats.
- 3-S could regulate the glucose tolerance effect.
- 3-S could significantly increase the insulin levels in experimentally induced rats.
- –OSO3− at the C3-position of chitosan is a key active site.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Triplitt, C.L.; Reasner, C.A.; Isley, W.L. Diabetes Mellitus. In Pharmacotherapy: A Pathophysiologic Approach; DiPiro, J.T., Talbert, R.L., Yee, G.C., Matzke, G.R., Wells, B.G., Posey, L.M., Eds.; McGraw-Hill Medical Publishing Division: New York, NY, USA, 2005; p. 1334. [Google Scholar]
- Kameswararao, B.; Kesavulu, M.M.; Apparao, C. Evaluation of antidiabetic effect of momordica cymbelaria fruit in alloxan-diabetic rats. Fitoterapia 2003, 74, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Pepato, M.T.; Mori, D.M.; Baviera, A.M.; Harami, J.B.; Vendramini, R.C.; Brunetti, I.L. Fruit of the Jambolan tree and experimental diabetes. J. Ethnopharmacol. 2005, 96, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Rosak, C. The pathophysiologic basis of efficacy and clinical experience with the new oral antidiabetic agents. J. Diabetes Complicat. 2002, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Q.; Zaman, K. Medicinal plants with hypoglycaemic activity. J. Ethnopharmacol. 1989, 26, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, K.R.; Mallikarjuna, K.; Nishanth, K.; Kuo, C.H.; Reddy, K.S. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2011, 124, 1436–1442. [Google Scholar] [CrossRef]
- Suba, V.; Murugesan, T.; Arunachalam, G.; Mardal, S.C.; Sahu, B.P. Anti-diabetic potential of barleria lupilina extract in rats. Phytomedicine 2004, 11, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Bing, L.; Wanshun, L.; Baoqin, H. Antidiabetic effects of Chitooligo-saccharides on pancreatic islet cells and streptozotocin induced diabetic rats. World J. Gastroenterol. 2007, 13, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.I.; Kai, H.; Shimada, K.; Yoshida, T.; Tokura, S.; Kurita, K. Regioselective syntheses of sulfated polysaccharides: Specific anti-HIV-1 activity of novel chitin sulfates. Carbohydr. Res. 1998, 306, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabeties and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Rudge, M.V.C.; Damasceno, D.C.; Volpato, G.T.; Almeida, F.C.G.; Calderon, I.M.P.; Lemonica, I.P. Effect of Ginkgo biloba on the reproductive outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. Braz. J. Med. Biol. Res. 2007, 40, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Oxidative stress as a mechanism of diabetes in diabetic BB prone rats: Effect of secoisolariciresinol diglucoside (SDG). Mol. Cell. Biochem. 2000, 209, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kamalakkannan, N.; Stanely Mainzen Prince, P. Rutin improves the antioxidant status in streptozotocin-induced diabetic rat tissues. Mol. Cell. Biochem. 2006, 293, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.E.; Yu, H.H.; Liu, S.; Zhang, W.W.; Zhang, Q.B.; Li, P.C. Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorganic Med. Chem. 2005, 13, 1387–1392. [Google Scholar] [CrossRef]
- Jelodar, G.; Mohsen, M.; Shahram, S. Effect of walnut leaf, coriander and pomegranate on blood glucose and his topathology of pancreas of alloxan-induced diabetic rats. Afr. J. Tradit. Complement. Altern. Med. 2003, 3, 299–305. [Google Scholar]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Hamden, K.; Carreau, S.; Lajmi, S.; Aloulou, D.; Kchaou, D.; Elfeki, A. Protective effect of 17 β-estradiol on hyperglycemia, stress oxidant, liver dysfunction and histological changes induced by alloxan in male rat pancreas and liver. Steroids 2008, 94, 495–501. [Google Scholar] [CrossRef]
- Lazarow, A. Alloxan diabetes and mechanism of β-cell damage by chemical agents. In Experimental Diabetes; Lazarow, A., Ed.; Blackwell Scientific Publication: Oxford, UK, 1964; pp. 49–69. [Google Scholar]
- Colca, J.R.; Kotagel, N.; Brooks, C.L.; Lacy, P.E.; Landt, M.; Mc Danield, M.L. Alloxan inhibition of a Ca2+ and calmodulin-dependent protein kinase in pancreatic islets. J. Biol. Chem. 1983, 25, 7260–7263. [Google Scholar]
- Edem, D.O. Hypoglycemic effects of ethanolic extract of Aligator pear seed (Persea americana Mill) in rats. Eur. J. Sci. Res. 2009, 33, 669–678. [Google Scholar]
- Funom, M. Etiology and pathophysiology of diabetes mellitus. Available online: http://ezinearticles.com/?Etiology-and-Pathophysiology-of-Diabetes-Mellitus&id=4353837 (accessed on 24 May 2010).
- Matteucci, E.; Giampietro, O. Proposal open for discussion: Defining agreed diagnostic procedures in experimental diabetes research. J. Ethnopharmacol. 2008, 115, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Buccolo, G.; David, M. Quantitative determination of serum triglycerides by use of enzyme. Clin. Chem. 1973, 19, 476–482. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, R.; He, X.; Liu, S.; Yu, H.; Qin, Y.; Chen, X.; Li, K.; Li, R.; Li, P. Antidiabetic Activity of Differently Regioselective Chitosan Sulfates in Alloxan-Induced Diabetic Rats. Mar. Drugs 2015, 13, 3072-3090. https://doi.org/10.3390/md13053072
Xing R, He X, Liu S, Yu H, Qin Y, Chen X, Li K, Li R, Li P. Antidiabetic Activity of Differently Regioselective Chitosan Sulfates in Alloxan-Induced Diabetic Rats. Marine Drugs. 2015; 13(5):3072-3090. https://doi.org/10.3390/md13053072
Chicago/Turabian StyleXing, Ronge, Xiaofei He, Song Liu, Huahua Yu, Yukun Qin, Xiaolin Chen, Kecheng Li, Rongfeng Li, and Pengcheng Li. 2015. "Antidiabetic Activity of Differently Regioselective Chitosan Sulfates in Alloxan-Induced Diabetic Rats" Marine Drugs 13, no. 5: 3072-3090. https://doi.org/10.3390/md13053072