Lindgomycin, an Unusual Antibiotic Polyketide from a Marine Fungus of the Lindgomycetaceae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Strains KF970 and LF327

2.2. Metabolic Profiles of the Strains KF970 and LF327
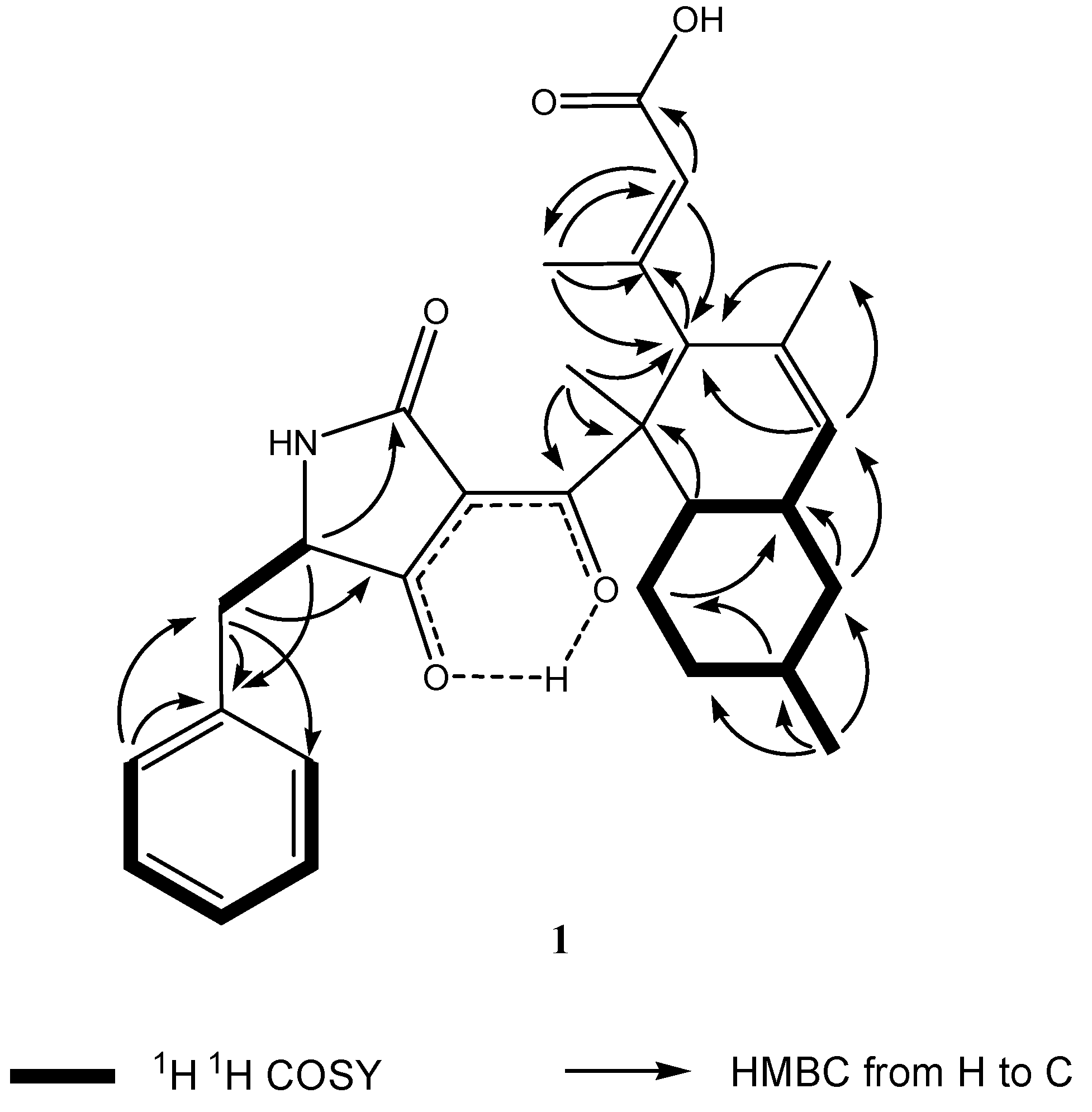
2.3. Structural Elucidation

| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC a,b, Mult. | δH c, Mult. (J in Hz) | δC a,b, Mult. | δH c, Mult. (J in Hz) | |
| 1 | 201.4, C | 202.3, C | ||
| 2 | 52.2, C | 52.7, C | ||
| 3α | 58.0, CH | 3.46, s | 57.9, CH | 3.52, s |
| 4 | 130.8, C | 130.9, C | ||
| 5 | 129.5, CH | 5.41, br s | 129.6, CH | 5.43, br s |
| 6α | 39.9, CH | 1.88, m | 39.8, CH | 1.90, m |
| 7α | 43.7, CH2 | 1.87, m | 43.8, CH2 | 1.88, m |
| 7β | 0.90, m | 0.86, m | ||
| 8α | 34.8, CH | 1.54, m | 34.8, CH | 1.56, m |
| 9α | 37.1, CH2 | 1.73, m | 37.1, CH2 | 1.67, m |
| 9β | 1.05, m | 1.06, m | ||
| 10α | 29.6, CH2 | 0.95, m | 29.6, CH2 | 1.07, m |
| 10β | 1.71, m | 1.76, m | ||
| 11β | 41.6, CH | 1.81, m | 41.8, CH | 1.87, m |
| 12 | 15.6, CH3 | 1.38, s | 15.6, CH3 | 1.49, s |
| 13 | 159.8, C | 159.9, C | ||
| 14 | 121.5, CH | 5.63, s | 121.6, CH | 5.66, s |
| 15 | 169.6, C | 169.6, C | ||
| 16 | 17.3, CH3 | 1.91, s | 17.2, CH3 | 1.97, s |
| 17 | 22.9, CH3 | 0.93, d (J = 6.5) | 22.9, CH3 | 0.92, d (J = 6.5) |
| 18 | 22.9, CH3 | 1.54, s | 22.8, CH3 | 1.55, s |
| 2′ | 173.0, C | 173.0, C | ||
| 3′ | n.d. | n.d. | ||
| 4′ | 194.1, C | 195.8, C | ||
| 5′ | 62.2, CH | 4.07, dd (J = 6.5, 4.3) | 60.4, CH | 3.90, m |
| 6′a | 38.5, CH2 | 3.09 dd (J = 13.9, 4.3) | 42.4, CH2 | 1.85, m |
| 6′b | 2.96 dd (J = 13.9 6.5) | 0.90, m | ||
| 7′ | 136.9, C | 22.1, CH | 1.50, m | |
| 8′ | 130.8, CH | 7.19, d (J = 8.0) | 23.9, CH3 | 0.97, d (J = 6.8) |
| 9′ | 129.3, CH | 7.24, t (J = 8.0) | 22.89, CH3 | 0,95, d (J = 6.8) |
| 10′ | 127.9, CH | 7.21, t (J = 8.0) | ||
| 11′ | 129.3, CH | 7.24, t (J = 8.0) | ||
| 12′ | 130.8, CH | 7.19, d (J = 8.0) | ||

2.4. Biological Activities
| Test Strain | 1 | 2 | Positive Controls |
|---|---|---|---|
| B. subtilis | 2.2 (±0.6) | 3.4 (±1.1) | chloramphenicol: 1.45 (±0.13) |
| X. campestris | 17.8 (±1.6) | 14.8 (±0.7) | chloramphenicol: 2.88 (±0.9) |
| S. epidermidis | 4.6 (±0.8) | 6.3 (±0.7) | chloramphenicol: 1.81 (±0.04) |
| S. aureus | 2.7 (±0.56) | 2.9 (±1.1) | chloramphenicol: 1.59 (±0.07) |
| S. aureus (MRSA) | 5.1 (±0.2) | 3.2 (±0.4) | chloramphenicol: 2.46 (±0.04) |
| C. albicans | 5.7 (±0.9) | 8.0 (±1.4) | nystatin: 1.71 (±0.28) |
| S. tritici | 5.1 (±0.7) | 10.0 (±3.1) | nystatin: 0.76 (±0.23) |
| P. acnes | 4.7 (±0.4) | 2.8 (±0.7) | chloramphenicol: 1.01 (±0.01) |
| E. coli | >100 | >100 | chloramphenicol: 373 (±0.10) |
| P. aeruginosa | >200 | >200 | chloramphenicol: 8.86 (±0.36) |
2.5. Biotechnological Scale Up
3. Experimental Section
3.1. General Experimental Procedures
3.2. Isolation, Cultivation, and Storage of the Producer Strains KF970 and LF327
3.3. Identification of the Strains KF970 and LF327
3.4. Comparison of the Metabolic Profiles the Strains KF970 and LF327
3.5. Fermentation and Production of Extracts for the Purification of Compounds 1 and 2
3.6. Scale up Production
3.7. Extraction and Isolation of Compounds 1 and 2
3.8. Antibiotic Activities Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef] [PubMed]
- Saleema, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Rotinsulu, H.; Kaneko, T.; Murakami, K.; Fujiwara, H.; Ukai, K.; Namikoshi, M. A new dibenz[b,e]oxepine derivative, 1-hydroxy-10-methoxy-dibenz[b,e]oxepin-6,11-dione, from a marine-derived fungus, Beauveria bassiana TPU942. Mar. Drugs 2012, 10, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ye, P.; Chen, C.-T.A.; Wang, K.; Liu, P.; He, S.; Wu, X.; Gan, L.; Ye, Y.; Wu, B. Two novel hepatocellular carcinoma cycle inhibitory cyclodepsipeptides from a hydrothermal vent crab-associated fungus Aspergillus clavatus C2WU. Mar. Drugs 2013, 11, 4761–4772. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, D.; Tao, M.; Chen, Y.; Dan, F.; Zhang, W. Scopararanes C–G: New oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella scoparia FS26. Mar. Drugs 2012, 10, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, X.; Sun, M.; Li, M. Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233. Mar. Drugs 2013, 11, 2713–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadury, P.; Mohammad, B.T.; Wright, P.C. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol. 2006, 33, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, K.; Tanaka, K.; Raja, H.A.; Miller, A.N.; Shearer, C.A. A molecular phylogenetic assessment of Massarina ingoldiana sensu lato. Mycologia 2010, 102, 729–746. [Google Scholar] [CrossRef] [PubMed]
- Raja, H.A.; Tanaka, K.; Hirayama, K.; Miller, A.N.; Shearer, C.A. Freshwater ascomycetes: Two new species of Lindgomyces (Lindgomycetaceae, Pleosporales, Dothideomycetes) froma Japan and USA. Mycologia 2011, 103, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Raja, H.A.; Oberlies, N.H.; El-Elimat, T.; Miller, A.N.; Zelski, S.E.; Shearer, C.A. Lindgomyces angustiascus, (Lindgomycetaceae, Pleosporales, Dothideomycetes), a new lignicolous species from freshwater habitats in the USA. Mycoscience 2013, 54, 353–361. [Google Scholar] [CrossRef]
- Abdel-Aziz, F.A.; Abdel-Wahab, M.A. Lolia aquatica gen. et sp. nov. (Lindgomycetaceae, Pleosporales), a new coelomycete from freshwater habitats in Egypt. Mycotaxon 2010, 114, 33–42. [Google Scholar] [CrossRef]
- Zhang, H.; Hyde, K.D.; Mckenzie, E.H.C.; Bahkali, A.H.; Zhou, D. Sequence data reveals phylogenetic affinities of Acrocalymma aquatica sp. nov., Aquasubmersa mircensis gen. et sp. nov. and Clohesyomyces aquaticus (freshwater Coelomycetes). Cryptogam. Mycol. 2012, 33, 333–346. [Google Scholar] [CrossRef]
- Royles, B.J.L. Naturally occurring tetramic acids: Structure, isolation, and synthesis. Chem. Rev. 1995, 95, 1981–2001. [Google Scholar] [CrossRef]
- Marfori, E.C.; Bamba, T.; Kajiyama, S.; Fukusaki, E.; Kobayashi, A. Biosynthetic studies of the tetramic acid antibiotic trichosetin. Tetrahedron 2002, 58, 6655–6658. [Google Scholar] [CrossRef]
- Onydeka, J.G.; Smith, S.K.; Zink, D.L.; Vicente, F.; Basilio, A.; Bills, G.F. Isolation, structure elucidation and antibacterial activity of a new tetramic acid, ascosetin. J. Antibiot. 2014, 67, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Slippers, B.; Wingfield, M.J.; Rheeder, J.; Marasas, W.F.O.; Philips, A.J.L.; Alves, A.; Burgess, T.; Barber, P.; Groenewald, J.Z. Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 2006, 55, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Zink, D.L.; Goetz, M.A.; Dombrowski, A.W.; Polishook, J.D.; Hazuda, D.J. Equisetin and a novel opposite stereochemical homolog phomasetin, two fungal metabolites as inhibitors of HIV-1 integrase. Tetrahedron Lett. 1998, 39, 2243–2246. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Kanamaru, N.; Morisaki, N.; Seto, H. Structure of lydicamycin, a new antibiotic of a novel skeletal type. Tetrahedron Lett. 1991, 32, 213–216. [Google Scholar] [CrossRef]
- Phillips, N.J.; Goodwin, J.T.; Fraiman, A.; Cole, R.J.; Lynn, D.G. Characterization of the fusarium toxin equisetin: The use of phenylboronates in structure assignment. J. Am. Chem. Soc. 1989, 111, 8223–8231. [Google Scholar] [CrossRef]
- Kattner, G. Die Expedition ARKTIS VIII/1 mit FS “Polarstern” 1991. In Berichte zur Polar-und Meeresforschung Expedition Reports; Alfred Wegener Institute for Polar and Marine Research: Bremerhaven, Germany, 1992; Volume 113, pp. 1–75. [Google Scholar]
- Wickerham, L.J. Taxonomy of Yeasts; Technical Bulletin No. 1029; United States Department of Agriculture: Washington, DC, USA, 1951.
- Wiese, J.; Ohlendorf, B.; Blümel, M.; Schmaljohann, R.; Imhoff, J.F. Phylogenetic identification of fungi isolated from the marine sponge Tethya aurantium and identification of their secondary metabolites. Mar. Drugs 2011, 9, 561–585. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.C.M.; Fagbola, O.; Costa, R.; Rumjanek, N.G.; Buchner, A.; Mendona-Hagler, L.; Smalla, K. Dynamics of fungal communities in bulk and maize rhizosphere soil in the tropics. Appl. Environ. Microbiol. 2003, 69, 3758–3766. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [PubMed]
- Aime, M.C. Generic Concepts in the Crepidotaceae as Inferred from Nuclear Large Subunit Ribosomal DNA Sequences, Morphology, and Basidiospore Dormancy Patterns. Ph.D. Thesis, University of Virginia, Charlottesville, VA, USA, 1999. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tatsuova, T.; Madden, T.L. Blast 2 sequences—A new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 1999, 174, 247–250. [Google Scholar] [CrossRef]
- Ohlendorf, B.; Schulz, D.; Erhard, A.; Nagel, K.; Imhoff, J.F. Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a Streptomyces species. J. Nat. Prod. 2012, 75, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.; Ohlendorf, B.; Labes, A.; Erhard, A.; Imhoff, J.F. Calcarides A–E, antibacterial macrocyclic and linear polyesters from a Calcarisporium strain. Mar. Drugs 2013, 11, 3309–3323. [Google Scholar] [CrossRef] [PubMed]
- Jansen, N.; Ohlendorf, B.; Erhard, A.; Imhoff, J.F. Helicusin E, Isochromophilone X and isochromophilone XI: New chloroazaphilones produced by the fungus Bartalinia robillardoides strain LF550. Mar. Drugs 2013, 11, 800–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prachyawarakorn, V.; Mahidol, C.; Sureram, S.; Sangpetsiripan, S.; Wiyakrutta, S.; Ruchirawat, S.; Kittakoop, P. Diketopiperazines and phthalides from a marine derived fungus of the order pleosporales. Planta Med. 2008, 74, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Kasettrathat, C.; Ngamrojanavanich, N.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Cytotoxic and antiplasmodial substances from marine-derived fungi, Nodulisporium sp. and CRI247-01. Phytochemistry 2008, 69, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Suzumura, Y.; Tomoda, H.; Masuma, R.; Haneda, K.; Kishi, M.; Iwai, Y.; Omura, S. Xanthoquinodins, new anticoccidial agents produced by Humicola sp. Production, isolation and physico-chemical and biological properties. J. Antibiot. (Tokyo) 1993, 46, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Kanokmedhakul, S.; Kanokmedhakul, K.; Phonkerd, N.; Soytong, K.; Kongsaeree, P.; Suksamrarn, A. Antimycobacterial anthraquinone-chromanone compound and diketopiperazine alkaloid from the fungus Chaetomium globosum KMITL-N0802. Planta Med. 2002, 68, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; She, Z.; Shao, C.; Wen, L.; Liu, F.; Zheng, Z.; Lin, Y. 1H and 13C NMR signal assignments of paecilin A and B, two new chromone derivatives from mangrove endophytic fungus Paecilomyces sp. (tree 1–7). Magn. Reson. Chem. 2007, 45, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Marlow, W.; Whalley, W.B.; Borthwick, A.D.; Bowden, R. The chemistry of fungi. Part LXV. The structures of ergochrysin A isoergochrysin A, and ergoxanthin, and of secalonic acids A, B, C, and D. J. Chem. Soc. C Org. 1971, 21, 3580–3590. [Google Scholar] [CrossRef]
- Zhang, W.; Krohn, K.; Zia-Ullah; Flörke, U.; Pescitelli, G.; Di Bari, L.; Antus, S.; Kurtán, T.; Rheinheimer, J.; Draeger, S.; Schulz, B. New mono- and dimeric members of the secalonic acid family: Blennolides A–G isolated from the fungus Blennoria sp. Chemistry 2008, 14, 4913–4923. [Google Scholar] [CrossRef] [PubMed]
- Lowery, C.A.; Park, J.; Gloeckner, C.; Meijler, M.M.; Mueller, R.S.; Boshoff, H.I.; Ulrich, R.L.; Barry III, C.E.; Bartlett, D.H.; Kravchenko, V.V.; et al. Defining the mode of action of tetramic acid antibacterials derived from Pseudomonas aeruginosa quorum sensing signals. J. Am. Chem. Soc. 2009, 131, 14473–14479. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. European Strategic Action Plan on Antibiotic Resistance; EUR/RC61/14; WHO Regional Committee for Europe: Copenhagen, Denmark, 2011. [Google Scholar]
- Drebes, J.; Künz, M.; Pereira, C.A.; Betzel, C.; Wrenger, C. MRSA infections: From classical treatment to suicide drugs. Curr. Med. Chem. 2013, 21, 1809–1819. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2012, 2013. Annual Report of the European Antimicrobial Resistance Surveillance Network Web Site. Available online: http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2012.pdf (accessed on 21 July 2015).
- McDowell, A.; Patrick, S.; Eishi, Y.; Lambert, P.; Eady, A. Propionibacterium acnes in human health and disease. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fuso-Almeida, A.M.; Mendes Giannini, M.F.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Wiese, J.; Labes, A.; Kramer, A.; Schmaljohann, R.; Imhoff, J.F. Lindgomycin, an Unusual Antibiotic Polyketide from a Marine Fungus of the Lindgomycetaceae. Mar. Drugs 2015, 13, 4617-4632. https://doi.org/10.3390/md13084617
Wu B, Wiese J, Labes A, Kramer A, Schmaljohann R, Imhoff JF. Lindgomycin, an Unusual Antibiotic Polyketide from a Marine Fungus of the Lindgomycetaceae. Marine Drugs. 2015; 13(8):4617-4632. https://doi.org/10.3390/md13084617
Chicago/Turabian StyleWu, Bin, Jutta Wiese, Antje Labes, Annemarie Kramer, Rolf Schmaljohann, and Johannes F. Imhoff. 2015. "Lindgomycin, an Unusual Antibiotic Polyketide from a Marine Fungus of the Lindgomycetaceae" Marine Drugs 13, no. 8: 4617-4632. https://doi.org/10.3390/md13084617







