The Structure-Activity Relationship between Marine Algae Polysaccharides and Anti-Complement Activity
Abstract
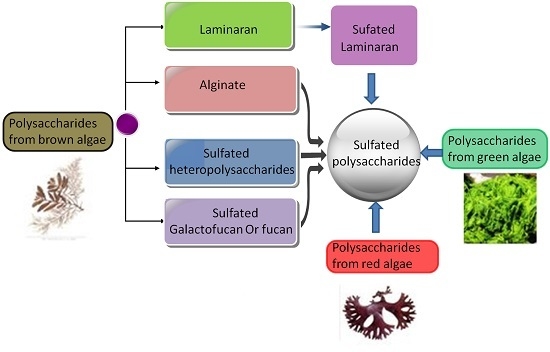
:1. Introduction
2. Results and Discussion
2.1. The Effect of the Extraction Methods on the Anti-Complement Activity
| Sample | Yields (%) | Fuc (%) | UA (%) | SO4 (%) | Monosaccharides (Molar Ratio) | Mw (kDa) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | Glc | Gal | Xyl | Fuc | ||||||
| SJW | 1.21 | 22.38 | 10.32 | 30.60 | 0.10 | 0.03 | 0.15 | 0 | 0.63 | 0.07 | 1 | 152.4 |
| SJW-1 | 10.67 | 15.84 | 33.36 | 15.05 | 0.55 | 0.10 | 0.96 | 0 | 1.22 | 0.37 | 1 | 77.8 |
| SJW-2 | 19.99 | 17.01 | 17.35 | 28.33 | 0.12 | 0.03 | 0.18 | 0 | 1.08 | 0.10 | 1 | 88.4 |
| SJW-3 | 37.89 | 28.96 | 0.45 | 35.10 | 0.02 | 0 | 0.02 | 0 | 0.27 | 0 | 1 | 162.7 |
| SJS | 1.08 | 34.97 | 3.44 | 36.88 | 0.06 | 0.03 | 0.06 | 0.01 | 0.11 | 0.02 | 1 | 106.3 |
| SJS-1 | 8.98 | 21.56 | 13.71 | 19.75 | 0.06 | 0.02 | 0.06 | 0.02 | 0.17 | 0.02 | 1 | 63.8 |
| SJS-2 | 15.89 | 31.07 | 8.21 | 34.84 | 0.08 | 0.03 | 0.07 | 0.01 | 0.28 | 0.03 | 1 | 84.5 |
| SJS-3 | 40.78 | 36.94 | 0 | 38.43 | 0.01 | 0 | 0.01 | 0 | 0.05 | 0 | 1 | 125.4 |
| HFW | 2.43 | 28.04 | 5.43 | 28.46 | 0.11 | 0 | 0.08 | 0.62 | 0.27 | 0.09 | 1 | 118.3/3.9 |
| HFW-1 | 18.51 | - | - | - | - | - | - | 1 | - | - | - | 3.9 |
| HFW-2 | 18.16 | 22.78 | 17.64 | 17.33 | 0.48 | 0.30 | 0.27 | 0.29 | 0.23 | 0.42 | 1 | 116.7/5.1 |
| HFW-3 | 28.01 | 32.85 | 0 | 31.62 | 0.05 | 0.01 | 0.04 | 0.01 | 0.29 | 0.04 | 1 | 114.2 |
| HFS | 1.53 | 21.89 | 1.98 | 30.59 | 0.07 | 0.01 | 0.09 | 1.22 | 0.25 | 0.02 | 1 | 97.3/3.6 |
| HFS-1 | 16.24 | - | - | - | - | - | - | 1 | - | - | - | 3.4 |
| HFS-2 | 10.66 | 25.79 | 20.84 | 25.66 | 0.27 | 0.02 | 0.20 | 0.03 | 0.22 | 0.04 | 1 | 42.0/4.3 |
| HFS-3 | 9.72 | 40.84 | 0.98 | 39.85 | 0 | 0 | 0 | 0 | 0.27 | 0.01 | 1 | 99.2 |
| SJS-OS | 98.19 | 33.31 | 3.31 | 46.36 | 0.06 | 0.03 | 0.05 | 0.05 | 0.20 | 0.05 | 1 | 119.5 |
| SJS-DS | 65.41 | 41.20 | 6.11 | 18.12 | 0.05 | 0.03 | 0.05 | 0.03 | 0.18 | 0.05 | 1 | 117.5/33.4 |
| SJS-DS-OS | 110.1 | 24.24 | 3.79 | 35.30 | 0.05 | 0.02 | 0.04 | 0.03 | 0.20 | 0.05 | 1 | 123.6 |
| ANW | 25.08 | 4.16 | 27.36 | 0.05 | 0.03 | 0.09 | 0.29 | 0.09 | 0.06 | 1 | 122.1 | |
| SC | 5.78 | 21.80 | 6.54 | 28.15 | 0.15 | 0.32 | 0.13 | 0.22 | 0.28 | 0.08 | 1 | 111.9 |
| SJW-2-R | 38.99 | 18.56 | 9.86 | 29.63 | 0.10 | 0.03 | 0.06 | 0.10 | 1.05 | 0.07 | 1 | 79.6 |
| SJW-2-HJ | 10.89 | 14.35 | 29.36 | 14.63 | 0.46 | 0.10 | 0.50 | 0 | 2.10 | 0.20 | 1 | 50.4 |
| SJW-2-HW | 83.10 | 25.23 | 8.36 | 35.69 | 0.09 | 0.01 | 0.10 | 0 | 0.56 | 0.06 | 1 | 95.6 |
| HFW-1-S | 109.1 | - | - | 41.38 | - | - | - | 1 | - | - | - | 13.7 |
| ES | 23.19 | - | - | 26.33 | - | - | - | - | 1 | - | - | 147.9 |
| GF | 13.30 | - | - | 20.42 | - | - | - | - | 1 | - | - | 137.2 |
| GL | 30.28 | - | - | 2.74 | - | - | - | - | 1 | - | - | 146.0 |
| PY | 10.71 | - | - | 2.72 | - | - | - | - | 1 | - | - | 140.7 |
| EP | 19.53 | - | 26.77 | 18.09 | - | 1 | 0.37 | 0.13 | 0.06 | 0.31 | - | 189.3 |
| UP | 18.75 | 28.39 | 20.88 | - | 1 | 0.45 | 0.15 | 0.04 | 0.33 | - | 163.4 | |
| CR | 8.13 | - | - | 32.84 | - | 0.03 | - | - | 0.14 | 1 | - | 174.9 |
| CF | 7.89 | - | - | 23.54 | - | 0.05 | - | 0.40 | 1 | 0.50 | - | 173.4 |


2.2. The Effect of the Fractionations on the Anti-Complement Activity
2.3. The Effect of Molecular Weight on the Anti-Complement Activity

2.4. The Effect of the Molar Ratio of Galactose to Fucose on the Anti-Complement Activity
2.5. The Effect of Sulfate on the Anti-Complement Activity

2.6. The Effect of the Content of Uronic acid (UA) on the Anti-Complement Activity

2.7. The Effect of the Linkage of Sulfated Galactofucan on the Anti-Complement Activity
2.8. The Effect of the Branching of Polysaccharides on the Anti-Complement Activity
2.9. The Effect of the Type of Monosaccharides on the Anti-Complement Activity


3. Experimental Section
3.1. Materials
3.2. Extraction and Preparation of Polysaccharides
3.3. Preparation and Purification of Polysaccharides
3.4. Preparation of Desulfated Polysaccharides
3.5. Preparation of over-Sulfated Polysaccharides
3.6. Preparation of the Polysaccharides with Carboxyl Reduction
3.7. Compositional Analysis
3.8. Anti-Complement Activity
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sahu, A.; Lambris, J.D. Complement inhibitors: A resurgent concept in anti-inflammatory therapertics. Immunopharmacology 2000, 49, 133–148. [Google Scholar] [CrossRef]
- Morgan, B.P.; Harris, C.L. Complement therapertics: History and current progress. Mol. Immunol. 2003, 40, 159–170. [Google Scholar] [CrossRef]
- Maillet, F.; Maurice, P.; Jean, C.; Kazatchkine, M.D. Structure-function relationships in the inhibitory effect of heparin on complement activation: Independency of the anti-coagulant and anti-complementary sites on the heparin molecule. Mol. Immunol. 1988, 25, 917–923. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Zhang, J.; Chen, D. Isolation and characterization of an anti-complementary polysaccharide D3-S1 from the roots of Bupleurum smithii. Int. Immunopharmacol. 2007, 7, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.B.; Yin, X.X.; Yuan, G.F.; Chen, X.O. Chemical and biological characterization of polysaccharides from the bark of Avicennia marina. Eur. Food Res. Technol. 2015, 241, 17–25. [Google Scholar] [CrossRef]
- Samuelsena, A.B.; Lunda, I.; Djahromia, J.M.; Paulsena, B.S.; Wolda, J.K.; Knutsen, H.S. Structural features and anti-complementary activity of some heteroxylan polysaccharide fractions from the seeds of Plantago major L. Carbohydr. Polym. 1999, 38, 133–143. [Google Scholar] [CrossRef]
- Ni, F.; Liu, L.; Song, Y.; Wang, X.; Zhao, Y.; Huang, W.; Wang, Z.; Xiao, W. Anti-complementary phenolic acids from Lonicera japonica. China J. Chin. Mater. Med. 2015, 40, 269–274. [Google Scholar]
- Sun, Q.; Bao, J. Purification, cloning and characterization of a metalloproteinase from Naja atra venom. Toxicon 2015, 56, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Makrides, S.C. Therapeutic Inhibition of the Complement System. Pharmacological 1998, 50, 59–88. [Google Scholar]
- Xu, X.; Chen, L.; Zhao, X. Review on the research and development of anti-complementary agents from natural products. Nat. Prod. Res. Dev. 2015, 27, 355. [Google Scholar]
- Mauzac, M.; Maillet, F.; Jozefonvicz, J.; Kazatchkine, M.D. Anticomplementary activity of dextran derivatives. Biomaterials 1985, 6, 61–63. [Google Scholar] [CrossRef]
- Crepon, B.; Maillet, F.; Kazatchkine, M.D.; Jozefonvicz, J. Molecular weight dependency of the acquired anticomplementary and anticoagulant activities of specifically substituted dextrans. Biomaterials 1987, 8, 248–253. [Google Scholar] [CrossRef]
- Blondin, C.; Chaubet, F.; Nardella, A.; Sinquin, C.; Jozefonvicz, J. Relationships between chemical characteristics and anticomplementary activity of fucans. Biomaterials 1996, 17, 597–603. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, W.; Sun, D.; Zhao, L.; Wang, J.; Duan, D.; Zhang, Q. Structural analysis and anti-complement activity of polysaccharides from Kjellmaniella crsaaifolia. Mar. Drugs 2015, 13, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Blondin, C.; Fisher, E.; Boisson-Vadal, C.; Kazatchkine, M.D.; Jozefonvicz, J. Inhibition of complement activation by natural sulfated polysaccharides (fucans) from brown seaweed. Mol. Immunol. 1994, 31, 247–253. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Kusaykin, M.; Bakunina, I.; Sova, V.; Ermakova, S.; Kuznetsova, T.; Besednova, N.; Zaporozhets, T.; Zvyagintseva, T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol. J. 2008, 3, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Mourao, P.A.S. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J. Appl. Phycol. 2001, 13, 173–184. [Google Scholar] [CrossRef]
- Pomin, V.H. Structural and functional insights into sulfated galactans: A systematic review. Glycoconj. J. 2010, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- USov, A.I. Structural analysis of red seaweed galactans of agar and carrageenan groups. Food Hydrocoll. 1998, 12, 301–308. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, W.; Wang, J.; Ren, S.; Song, N.; Duan, D.; Zhang, Q. Characterization of laminaran and a highly sulfated polysaccharide from Sargassum fusiforme. Carbohydr. Res. 2014, 385, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, W.; Wang, J.; Ren, S.; Song, N.; Zhang, Q. Structural analysis of heteropolysaccharide from Saccharina japonica and its derived oligosaccharides. Int. J. Biol. Macromol. 2013, 62, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Guo, Z.; Wang, J.; Zhang, W.; Zhang, Q. Structural analysis of sulfated fucan from Saccharina japonica by electrospray ionization tandem mass spectrometry. Carbohydr. Res. 2013, 369, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, J.; Ren, S.; Song, N.; Zhang, Q. Structural analysis of a heteropolysaccharide from Saccharina japonica by electrospray mass spectrometry in tandem with collision-induced dissociation tandem mass spectrometry (ESI-CID-MS/MS). Mar. Drugs 2012, 10, 2138–2152. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, Q.; Wang, J.; Zhang, W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, P.; Pujol, C.A.; Damonte, E.B.; Ghosh, T.; Ray, B. Polysaccharides from Padina tetrastromatica: Structural features, chemical modification and antiviral activity. Carbohydr. Polym. 2010, 80, 513–520. [Google Scholar] [CrossRef]
- Pereira, M.S.; Melo, F.R.; Mourão, P.A.S. Is there a correlation between structure and anticoagulant action of sulfated galactans and sulfated fucans? Glycobiology 2002, 12, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xue, X.J.; Sun, J.L.; Xu, S.Y. Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed Hizikia fusiforme. Carbohydr. Res. 2006, 341, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, X.; Lv, Y.; Liu, Y.; Lang, Y.; Wu, J.; Liu, X.; Li, M.; Yu, G. Analysis of structural heterogeneity of fucoidan from Hizikia fusiforme by ES-CID-MS/MS. Carbohydr. Polym. 2012, 90, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–156. [Google Scholar] [CrossRef]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouaultb, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Daniel, R.; Berteau, O.; Jozefonvicz, J.; Goasdoue, N. Degradation of algal (Ascophyllum nodosum) fucoidan by an enzymatic activity contained in digestive glands of the marine mollusc Pecten maximus. Carbohydr. Res. 1999, 322, 291–297. [Google Scholar] [CrossRef]
- Daniel, R.; Berteau, O.; Chevolot, L.; Varenne, A.; Gareil, P.; Goasdoue, N. Regioselective desulfation of sulfated l-fucopyranoside by a new sulfoesterase from the marine mollusk Pecten maximus. Application to the structural study of algal fucoidan (Ascophyllum nodosum). Eur. J. Biochem. 2001, 268, 5617–5626. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Tang, Q.; Li, D.; Wu, X.; Wang, J. Isolation and characterization of a sea cucumber fucoidan-utilizing marine bacterium. Lett. Appl. Microbiol. 2010, 50, 301–307. [Google Scholar]
- Clement, M.J.; Tissot, B.; Chevolot, L.; Adjadj, E.; Du, Y.; Curmi, P.A.; Daniel, R. NMR characterization and molecular modeling of fucoidan showing the importance of oligosaccharide branching in its anticomplementary activity. Glycobiology 2010, 20, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Nifantiev, N.; Ustyuzhanina, N.; Krylov, V.; Grachev, A.; Gerbst, A. Synthesis, NMR and conformational studies of fucoidan fragments, 8: Convergent synthesis of branched and linear oligosaccharides. Synthesis 2006, 23, 4017–4031. [Google Scholar] [CrossRef]
- Kariya, Y.; Watabe, S.; Kyogashima, M.; Ishihara, M.; Ishii, T. Structure of fucose branches in the glycosaminoglycan from the body wall of the sea cucumber Stichopus japonicus. Carbohydr. Res. 1997, 297, 273–279. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Wang, J.; Shi, X.; Zhang, Z. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chin. J. Oceanol. Limnol. 2009, 27, 1–5. [Google Scholar] [CrossRef]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Klerx, J.P.A.M.; Beukelman, C.J.; Dijk, H.V.; Willers, J.M.N. Microassay for colorimetric estimation of complement activity in guinea pig, human and mouse serum. J. Immunol. Methods 1983, 63, 215–220. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Zhang, W.; Liang, H.; Zhang, Q. The Structure-Activity Relationship between Marine Algae Polysaccharides and Anti-Complement Activity. Mar. Drugs 2016, 14, 3. https://doi.org/10.3390/md14010003
Jin W, Zhang W, Liang H, Zhang Q. The Structure-Activity Relationship between Marine Algae Polysaccharides and Anti-Complement Activity. Marine Drugs. 2016; 14(1):3. https://doi.org/10.3390/md14010003
Chicago/Turabian StyleJin, Weihua, Wenjing Zhang, Hongze Liang, and Quanbin Zhang. 2016. "The Structure-Activity Relationship between Marine Algae Polysaccharides and Anti-Complement Activity" Marine Drugs 14, no. 1: 3. https://doi.org/10.3390/md14010003