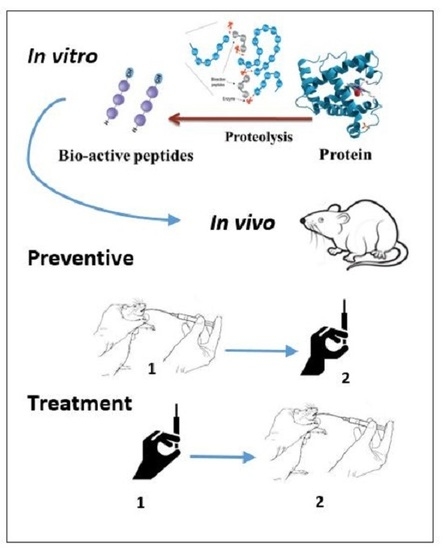
Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Hypertensive Effect of Protein Hydrolysate from Actinopyga lecanora (Sea Cucumber) in Rats
Abstract
:1. Introduction
2. Results and Discussion
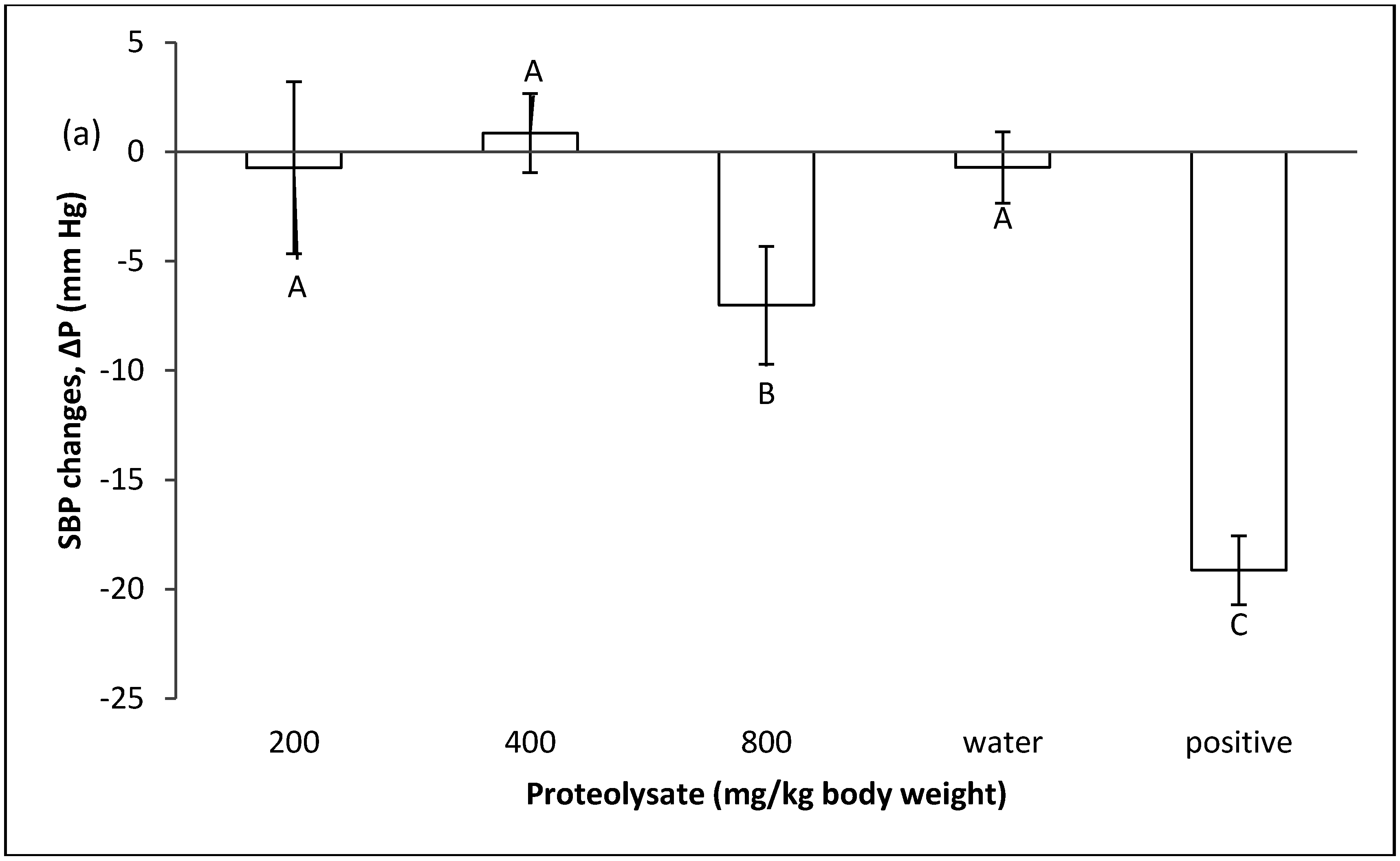
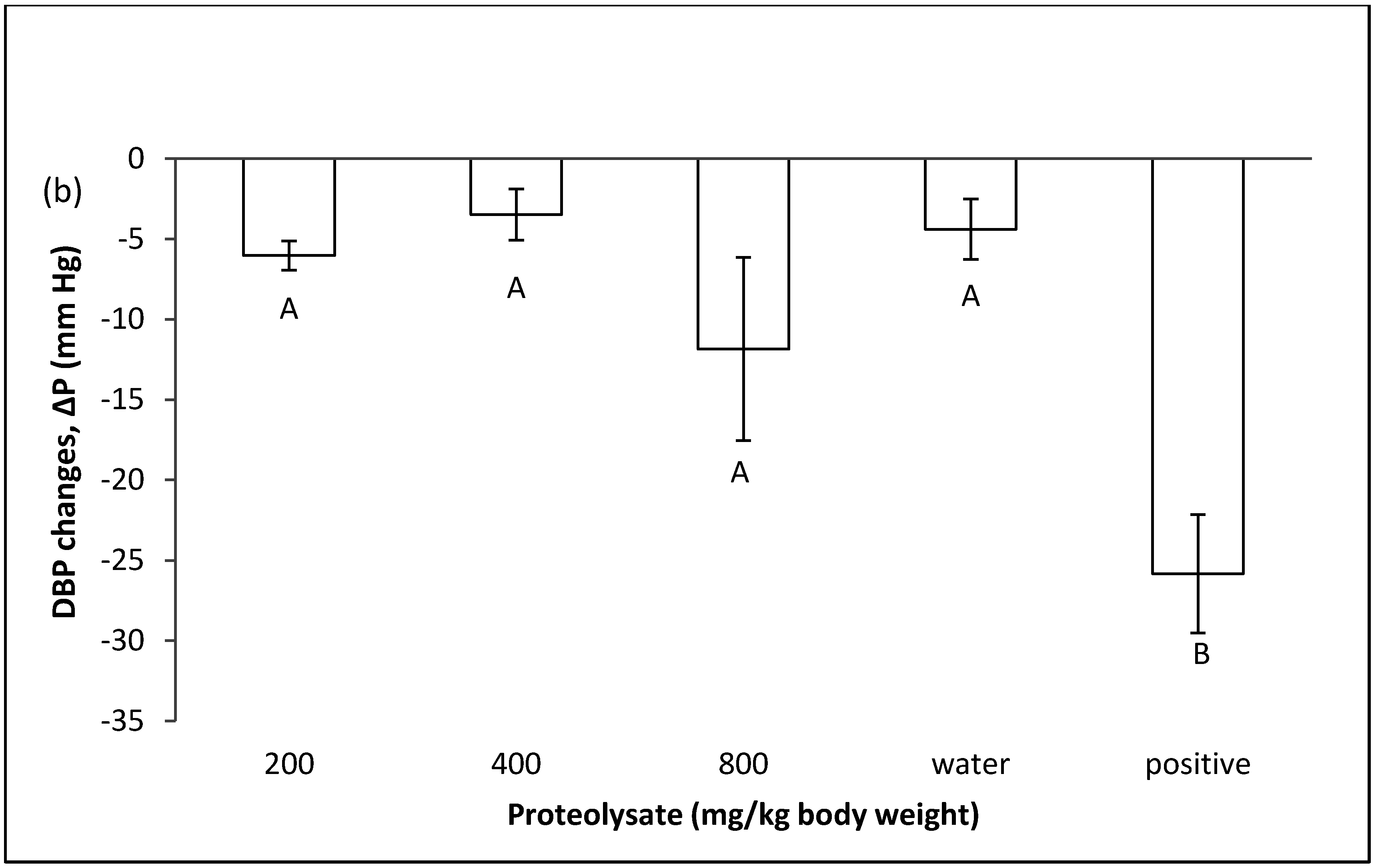
2.1. Effect of Proteolysate on Normal Blood Pressure
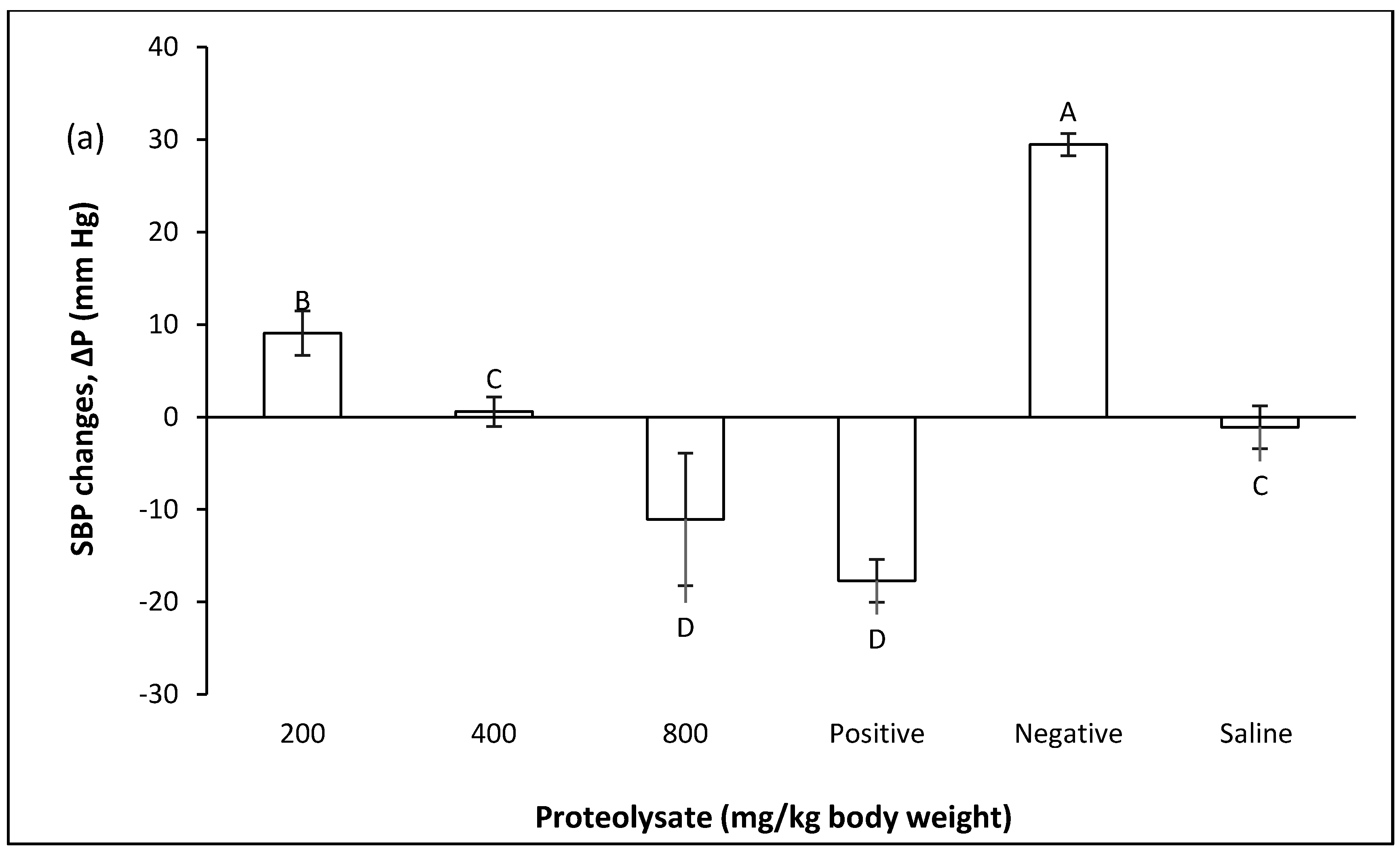
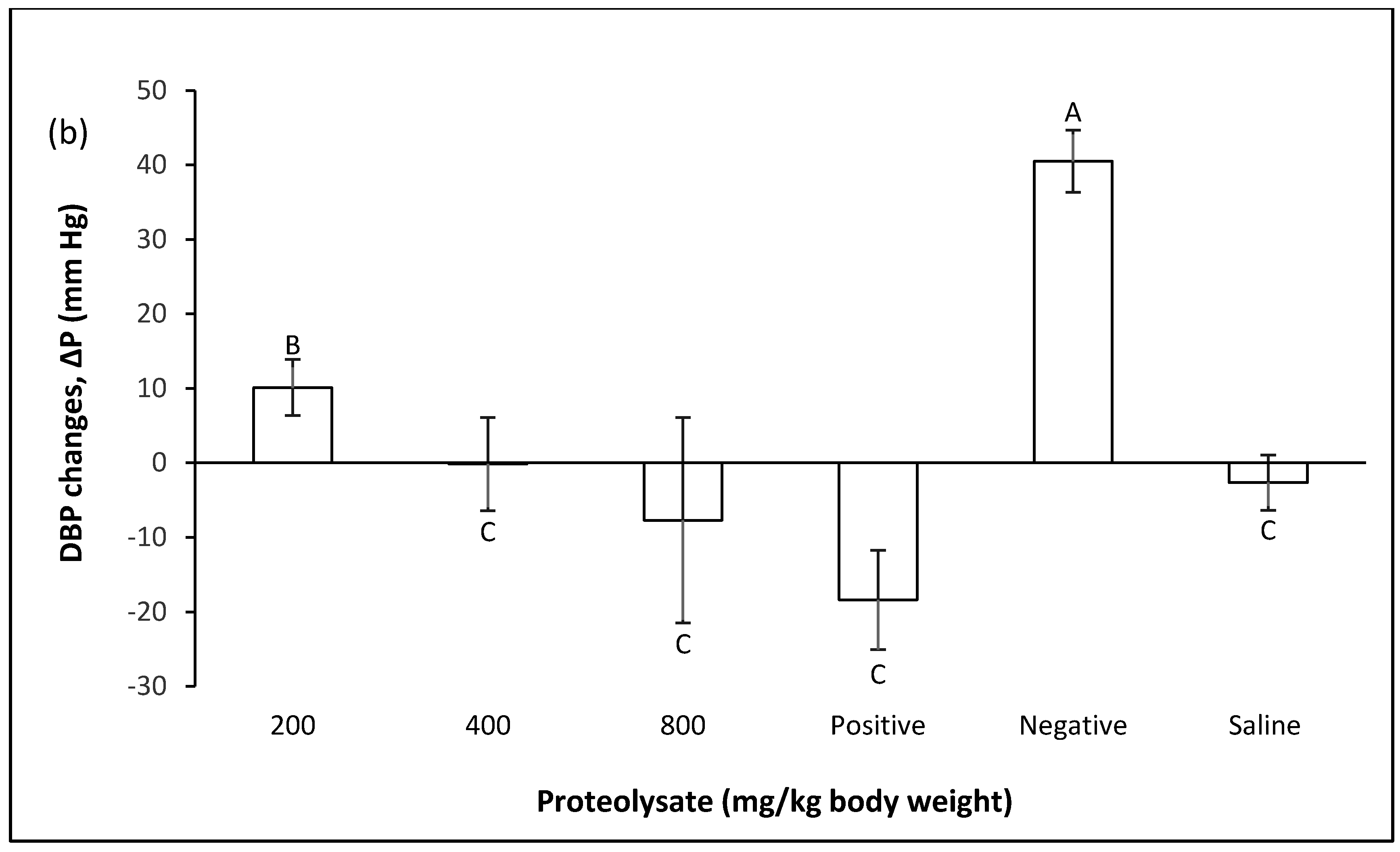
2.2. Preventive Group
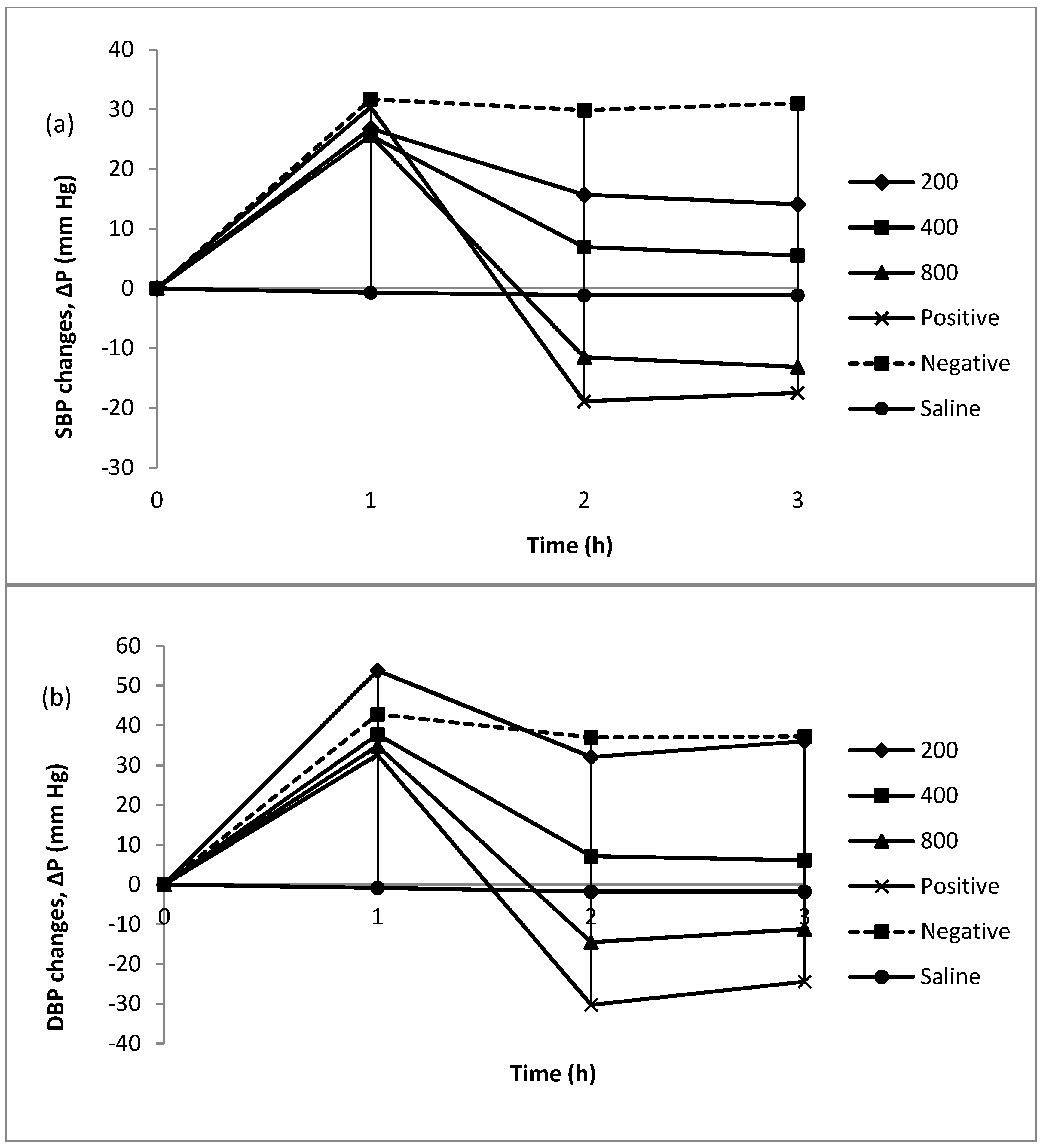
2.2.1. Changes in Blood Pressure in Pre-Fed Rats
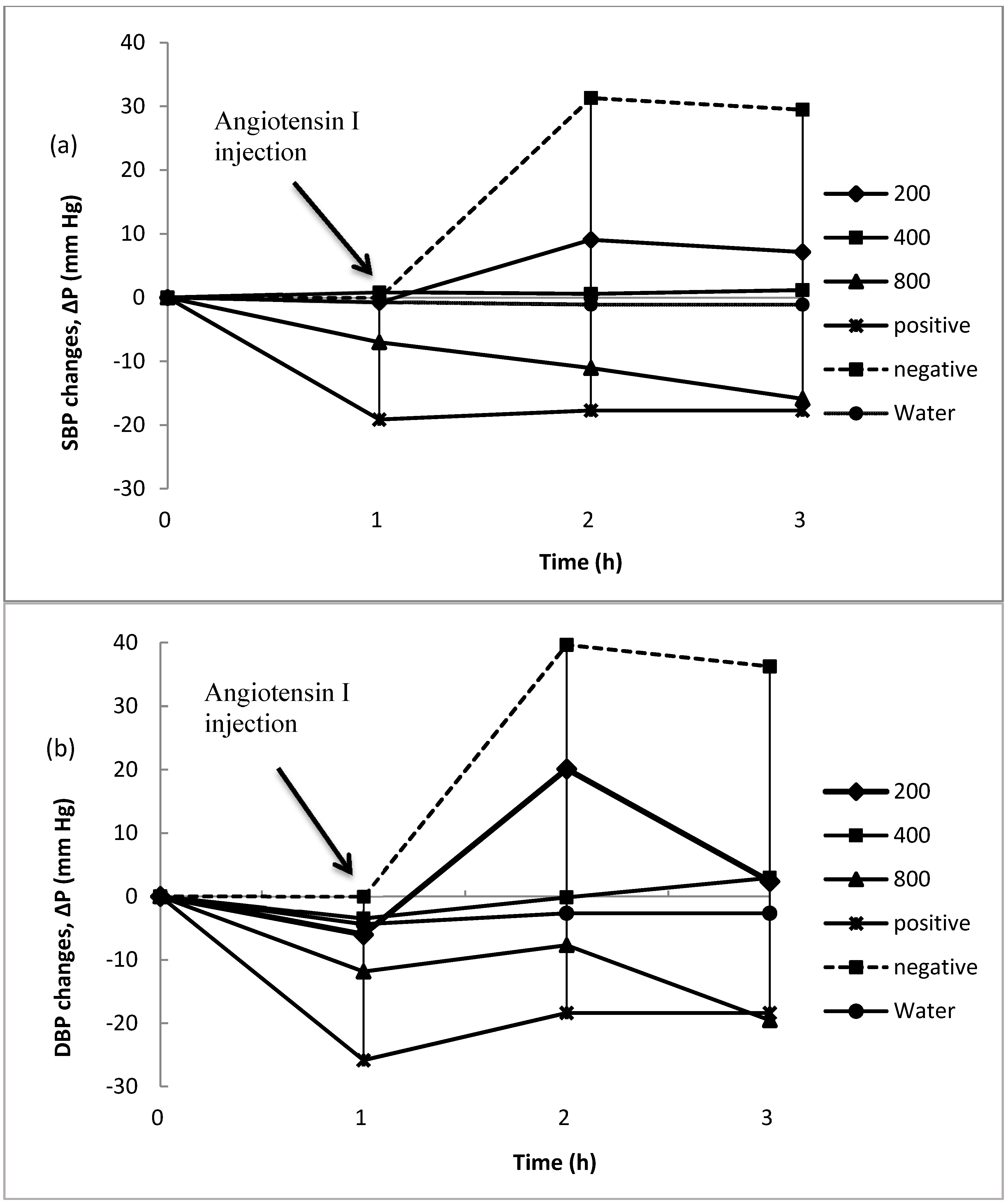
2.2.2. Effect of Proteolysates in the Prevention of Blood Pressure Increases after Inducing Hypertension
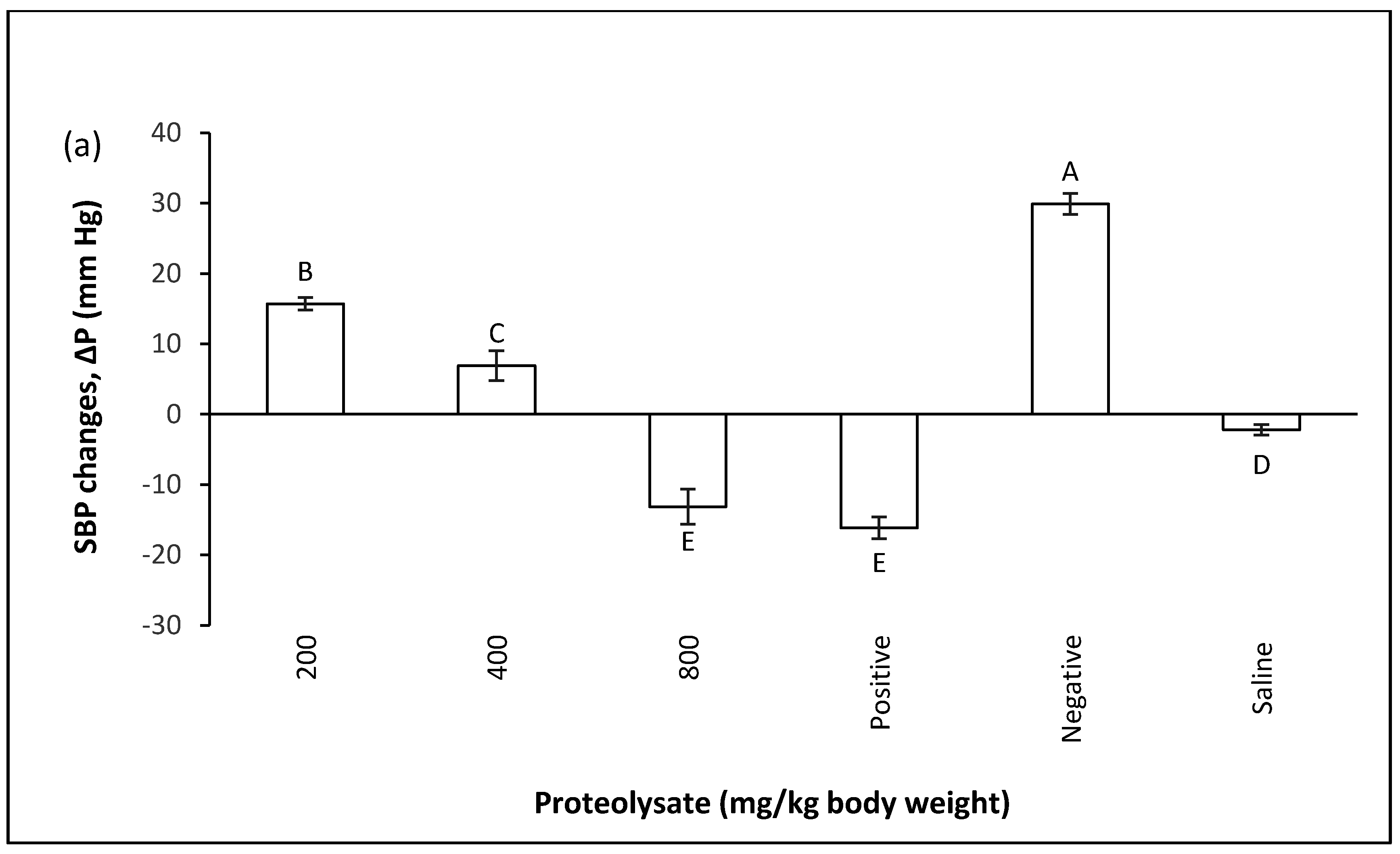
2.3. Treatment Group
2.3.1. Changes in Blood Pressure after Inducing Hypertension
2.3.2. Curative Potential of Proteolysate
2.4. Heart Rate
3. Materials and Methods
3.1. Material
3.2. Animals
3.3. Preparation of Proteolysate
3.4. In Vivo Study
3.5. Effect of Proteolysate on ACE in Preventive Group
3.6. Effect of Proteolysate on High Blood Pressure in Treatment Group
3.7. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviation
| ACE | Angiotensin converting enzyme |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| HR | Heart rate |
| CVD | Cardiovascular disease |
| SD | Sprague dawley |
References
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.; Ezzati, M. The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Risks-Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Yadav, S.; Boddula, R.; Genitta, G.; Bhatia, V.; Bansal, B.; Kongara, S.; Ramesh, V. Prevalence & risk factors of pre-hypertension & hypertension in an affluent north Indian population. Indian J. Med. Res. 2008, 128, 712–720. [Google Scholar] [PubMed]
- Rampal, L.; Rampal, S.; Azhar, M.; Rahman, A.R. Prevalence, awareness, treatment and control of hypertension in Malaysia: A national study of 16,440 subjects. Public Health 2008, 122, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Jao, C.L.; Huang, S.L.; Hsu, K.C. Angiotensin I-converting enzyme inhibitory peptides: Inhibition mode, bioavailability, and antihypertensive effects. BioMedicine 2012, 2, 130–136. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Sharma, R.; Baruwa, A. Pharmacological review on natural ACE inhibitors. Der Pharm. Lett. 2010, 2, 273–293. [Google Scholar]
- Li, G.H.; Qu, M.R.; Wan, J.Z.; You, J.M. Antihypertensive effect of rice protein hydrolysate with angiotensin I-converting enzyme inhibitory activity in spontaneously hypertensive rats. Asia Pac. J. Clin. Nutr. 2007, 16, 275–280. [Google Scholar] [PubMed]
- Brunner, H.R.; Laragh, J.H.; Baer, L.; Newton, M.A.; Goodwin, F.T.; Krakoff, L.R.; Bühler, F.R. Essential hypertension: Renin and aldosterone, heart attack and stroke. N. Engl. J. Med. 1972, 286, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Contreras, M.; Recio, I.; Aleixandre, A. ACE-inhibitory and antihypertensive properties of a bovine casein hydrolysate. Food Chem. 2009, 112, 211–214. [Google Scholar] [CrossRef]
- Je, J.Y.; Park, P.J.; Byun, H.G.; Jung, W.K.; Kim, S.K. Angiotensin I converting enzyme (ACE) inhibitory peptide derived from the sauce of fermented blue mussel, Mytilus edulis. Bioresour. Technol. 2005, 96, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Ma, H.; Pan, Z.; Luo, L.; Wang, Z.; He, R. Preparation and antihypertensive activity of peptides from Porphyra yezoensis. Food Chem. 2010, 123, 14–20. [Google Scholar] [CrossRef]
- Ghanbari, R.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Actinopyga lecanora hydrolysates as natural antibacterial agents. Int. J. Mol. Sci. 2012, 13, 16796–16811. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Zhang, C.; Liu, C. Angiotensin converting enzyme (ACE) inhibitory, antihypertensive and antihyperlipidaemic activities of protein hydrolysates from Rhopilema esculentum. Food Chem. 2012, 134, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Zhu, Y.J.; Shen, J.Y. Progress of Antihypertensive Peptide Research. Food Ferment. Ind. 2006, 6, 85–90. [Google Scholar]
- Fuglsang, A.; Rattray, F.P.; Nilsson, D.; Nyborg, N.C. Lactic acid bacteria: Inhibition of angiotensin converting enzyme in vitro and in vivo. Antonie van Leeuwenhoek 2003, 83, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chalé, F.G.H.; Ruiz, J.C.R.; Fernández, J.J.A.; Ancona, D.A.B.; Campos, M.R.S. ACE inhibitory, hypotensive and antioxidant peptide fractions from Mucuna pruriens proteins. Process Biochem. 2014, 49, 1691–1698. [Google Scholar] [CrossRef]
- Fritz, M.; Vecchi, B.; Rinaldi, G.; Añón, M.C. Amaranth seed protein hydrolysates have in vivo and in vitro antihypertensive activity. Food Chem. 2011, 126, 878–884. [Google Scholar] [CrossRef]
- Lima-Landman, M.; Borges, A.; Cysneiros, R.; de Lima, T.; Souccar, C.; Lapa, A. Antihypertensive effect of a standardized aqueous extract of Cecropia glaziovii Sneth in rats: An in vivo approach to the hypotensive mechanism. Phytomedicine 2007, 14, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Mori, T.; Kawahara, S.; Miake, K.; Kodama, Y.; Sugiyama, M.; Muguruma, M. Angiotensin-I Converting Enzyme Inhibitory Peptide Derived from Porcine Skeletal Muscle Myosin and Its Antihypertensive Activity in Spontaneously Hypertensive Rats. J. Food Sci. 2007, 72, S702–S706. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Ogura, K.; Miyakoshi, M.; Ishii, F.; Kawanishi, H.; Kurumazuka, D.; Moriguchi, S. Antihypertensive effect of angiotensin I-converting enzyme inhibitory peptides from a sesame protein hydrolysate in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 70, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Yamagami, T.; Ohshima, K. Effects of an ACE-inhibitory agent, katsuobushi oligopeptide, in the spontaneously hypertensive rat and in borderline and mildly hypertensive subjects. Nutr. Res. 2001, 21, 1149–1158. [Google Scholar] [CrossRef]
- Iroyukifujita, H.; Eiichiyokoyama, K.; Yoshikawa, M. Classification and Antihypertensive Activity of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Food Proteins. J. Food Sci. 2000, 65, 564–569. [Google Scholar] [CrossRef]
- Matsui, T.; Imamura, M.; Oka, H.; Osajima, K.; Kimoto, K.I.; Kawasaki, T.; Matsumoto, K. Tissue distribution of antihypertensive dipeptide, Val-Tyr, after its single oral administration to spontaneously hypertensive rats. J. Pept. Sci. 2004, 10, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nakamura, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Isolation and structural analysis of antihypertensive peptides that exist naturally in Gouda cheese. J. Dairy Sci. 2000, 83, 1434–1440. [Google Scholar] [CrossRef]
- Suetsuna, K.; Nakano, T. Identification of an antihypertensive peptide from peptic digest of wakame Undaria pinnatifida. J. Nutr. Biochem. 2000, 11, 450–454. [Google Scholar] [CrossRef]
- Matsui, T.; Tamaya, K.; Seki, E.; Osajima, K.; Matsumoto, K.; Kawasaki, T. Absorption of Val-Tyr with in vitro angiotensin I-converting enzyme inhibitory activity into the circulating blood system of mild hypertensive subjects. Biol. Pharm. Bull. 2002, 25, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Je, J.Y.; Park, J.Y.; Jung, W.K.; Park, P.J.; Kim, S.K. Isolation of angiotensin I converting enzyme (ACE) inhibitor from fermented oyster sauce, Crassostrea gigas. Food Chem. 2005, 90, 809–814. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Cheng, X.; Zhou, J.; Tang, X.; Mao, X.Y. Hypertension-attenuating effect of whey protein hydrolysate on spontaneously hypertensive rats. Food Chem. 2012, 134, 122–126. [Google Scholar] [CrossRef]
- Gillman, M.W.; Kannel, W.B.; Belanger, A.; D’Agostino, R.B. Influence of heart rate on mortality among persons with hypertension: The Framingham Study. Am. Heart J. 1993, 125, 1148–1154. [Google Scholar] [CrossRef]
- Boerth, R.C.; Covell, J.W.; Pool, P.E.; Ross, J. Increased myocardial oxygen consumption and contractile state associated with increased heart rate in dogs. Circ. Res. 1969, 24, 725–734. [Google Scholar] [CrossRef] [PubMed]

| Group | mg/kg Body Weight | HR Preventive Group (BPM) | HR Treatment Group (BPM) |
|---|---|---|---|
| Proteolysate concentration | 200 | 363.06 ± 25.51 | 333.00 ± 6.56 |
| Proteolysate concentration | 400 | 368.00 ± 30.81 | 365.33 ± 18.90 |
| Proteolysate concentration | 800 | 321.50 ± 90.06 | 358.67 ± 10.41 |
| Positive control | 50 | 339.33 ± 42.34 | 372.67 ± 23.03 |
| Negative control (AngiotensinI) | - | 332.42 ± 59.88 | 380.75 ± 10.63 |
| Negative control (Water) | - | 382.00 ± 10.15 | 338.67 ± 77.23 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadegh Vishkaei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Hypertensive Effect of Protein Hydrolysate from Actinopyga lecanora (Sea Cucumber) in Rats. Mar. Drugs 2016, 14, 176. https://doi.org/10.3390/md14100176
Sadegh Vishkaei M, Ebrahimpour A, Abdul-Hamid A, Ismail A, Saari N. Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Hypertensive Effect of Protein Hydrolysate from Actinopyga lecanora (Sea Cucumber) in Rats. Marine Drugs. 2016; 14(10):176. https://doi.org/10.3390/md14100176
Chicago/Turabian StyleSadegh Vishkaei, Mahdokht, Afshin Ebrahimpour, Azizah Abdul-Hamid, Amin Ismail, and Nazamid Saari. 2016. "Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Hypertensive Effect of Protein Hydrolysate from Actinopyga lecanora (Sea Cucumber) in Rats" Marine Drugs 14, no. 10: 176. https://doi.org/10.3390/md14100176